| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 1,3-beta-glucan synthase |
|---|
| Ligand | BDBM50100333 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_39039 |
|---|
| IC50 | 0.630000±n/a nM |
|---|
| Citation |  Masubuchi, K; Okada, T; Kohchi, M; Murata, T; Tsukazaki, M; Kondoh, O; Yamazaki, T; Satoh, Y; Ono, Y; Tsukaguchi, T; Kobayashi, K; Ono, N; Inoue, T; Horii, I; Shimma, N Synthesis and antifungal activities of novel 1,3-beta-D-glucan synthase inhibitors. Part 2. Bioorg Med Chem Lett11:1273-6 (2001) [PubMed] Masubuchi, K; Okada, T; Kohchi, M; Murata, T; Tsukazaki, M; Kondoh, O; Yamazaki, T; Satoh, Y; Ono, Y; Tsukaguchi, T; Kobayashi, K; Ono, N; Inoue, T; Horii, I; Shimma, N Synthesis and antifungal activities of novel 1,3-beta-D-glucan synthase inhibitors. Part 2. Bioorg Med Chem Lett11:1273-6 (2001) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 1,3-beta-glucan synthase |
|---|
| Name: | 1,3-beta-glucan synthase |
|---|
| Synonyms: | 1,3-beta-glucan synthase | Beta-1,3-glucan synthase | Glucan Synthase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 218006.59 |
|---|
| Organism: | Candida albicans (Yeast) |
|---|
| Description: | O13428 |
|---|
| Residue: | 1897 |
|---|
| Sequence: | MSYNDNNNHYYDPNQQGGMPPHQGGEGYYQQQYDDMGQQPHQQDYYDPNAQYQQQPYDMD
GYQDQANYGGQPMNAQGYNADPEAFSDFSYGGQTPGTPGYDQYGTQYTPSQMSYGGDPRS
SGASTPIYGGQGQGYDPTQFNMSSNLPYPAWSADPQAPIKIEHIEDIFIDLTNKFGFQRD
SMRNMFDYFMTLLDSRSSRMSPAQALLSLHADYIGGDNANYRKWYFSSQQDLDDSLGFAN
MTLGKIGRKARKASKKSKKARKAAEEHGQDVDALANELEGDYSLEAAEIRWKAKMNSLTP
EERVRDLALYLLIWGEANQVRFTPECLCYIYKSATDYLNSPLCQQRQEPVPEGDYLNRVI
TPLYRFIRSQVYEIYDGRFVKREKDHNKVIGYDDVNQLFWYPEGISRIIFEDGTRLVDIP
QEERFLKLGEVEWKNVFFKTYKEIRTWLHFVTNFNRIWIIHGTIYWMYTAYNSPTLYTKH
YVQTINQQPLASSRWAACAIGGVLASFIQILATLFEWIFVPREWAGAQHLSRRMLFLVLI
FLLNLVPPVYTFQITKLVIYSKSAYAVSIVGFFIAVATLVFFAVMPLGGLFTSYMNKRSR
RYIASQTFTANYIKLKGLDMWMSYLLWFLVFLAKLVESYFFSTLSLRDPIRNLSTMTMRC
VGEVWYKDIVCRNQAKIVLGLMYLVDLLLFFLDTYMWYIICNCIFSIGRSFYLGISILTP
WRNIFTRLPKRIYSKILATTEMEIKYKPKVLISQIWNAIVISMYREHLLAIDHVQKLLYH
QVPSEIEGKRTLRAPTFFVSQDDNNFETEFFPRNSEAERRISFFAQSLATPMPEPLPVDN
MPTFTVFTPHYSEKILLSLREIIREDDQFSRVTLLEYLKQLHPVEWDCFVKDTKILAEET
AAYENGDDSEKLSEDGLKSKIDDLPFYCIGFKSAAPEYTLRTRIWASLRSQTLYRTVSGF
MNYARAIKLLYRVENPELVQYFGGDPEGLELALERMARRKFRFLVSMQRLSKFKDDEMEN
AEFLLRAYPDLQIAYLDEEPALNEDEEPRVYSALIDGHCEMLENGRRRPKFRVQLSGNPI
LGDGKSDNQNHAVIFHRGEYIQLIDANQDNYLEECLKIRSVLAEFEEMNVEHVNPYAPNL
KSEDNNTKKDPVAFLGAREYIFSENSGVLGDVAAGKEQTFGTLFARTLAQIGGKLHYGHP
DFLNATFMLTRGGVSKAQKGLHLNEDIYAGMNAMMRGGKIKHCEYYQCGKGRDLGFGSIL
NFTTKIGAGMGEQMLSREYFYLGTQLPLDRFLSFYYGHPGFHINNLFIQLSLQVFILVLG
NLNSLAHEAIMCSYNKDVPVTDVLYPFGCYNIAPAVDWIRRYTLSIFIVFFISFIPLVVQ
ELIERGVWKAFQRFVRHFISMSPFFEVFVAQIYSSSVFTDLTVGGARYISTGRGFATSRI
PFSILYSRFADSSIYMGARLMLILLFGTVSHWQAPLLWFWASLSALMFSPFIFNPHQFAW
EDFFLDYRDFIRWLSRGNTKWHRNSWIGYVRLSRSRITGFKRKLTGDVSEKAAGDASRAH
RSNVLFADFLPTLIYTAGLYVAYTFINAQTGVTSYPYEINGSTDPQPVNSTLRLIICALA
PVVIDMGCLGVCLAMACCAGPMLGLCCKKTGAVIAGVAHGVAVIVHIIFFIVMWVTEGFN
FARLMLGIATMIYVQRLLFKFLTLCFLTREFKNDKANTAFWTGKWYNTGMGWMAFTQPSR
EFVAKIIEMSEFAGDFVLAHIILFCQLPLLFIPLVDRWHSMMLFWLKPSRLIRPPIYSLK
QARLRKRMVRKYCVLYFAVLILFIVIIVAPAVASGQIAVDQFANIGGSGSIADGLFQPRN
VSNNDTGNHRPKTYTWSYLSTRFTGSTTPYSTNPFRV
|
|
|
|---|
| BDBM50100333 |
|---|
| n/a |
|---|
| Name | BDBM50100333 |
|---|
| Synonyms: | CHEMBL2371765 | RO-09-3655 derivative |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C80H136N18O24 |
|---|
| Mol. Mass. | 1734.042 |
|---|
| SMILES | [H][C@@]12C[C@@H](O)CN1C(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](C)NC(=O)[C@@]([H])(NC(=O)C[C@@H](CCCCCCCCCCCCC)OC(=O)[C@H](CCCNC(=O)[C@@H](CCCN)N(CCN)CCN)NC(=O)[C@@]([H])(NC(=O)CNC(=O)[C@@]([H])(NC(=O)[C@]1([H])[C@@H](O)CCN1C(=O)[C@@]([H])(NC(=O)[C@@]([H])(NC2=O)[C@@H](C)O)[C@@H](C)O)[C@H](O)CC(N)=O)[C@@H](C)O)[C@@H](C)O)C(C)C |
|---|
| Structure | 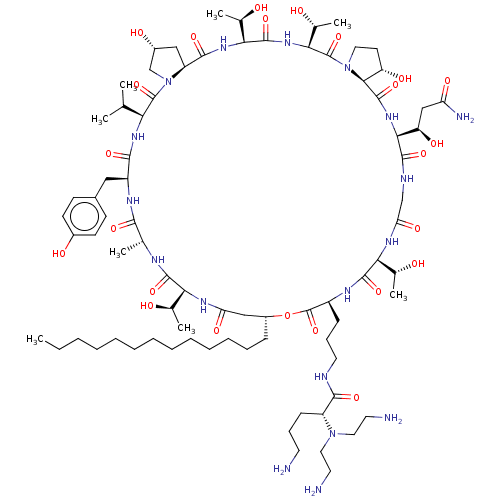
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Masubuchi, K; Okada, T; Kohchi, M; Murata, T; Tsukazaki, M; Kondoh, O; Yamazaki, T; Satoh, Y; Ono, Y; Tsukaguchi, T; Kobayashi, K; Ono, N; Inoue, T; Horii, I; Shimma, N Synthesis and antifungal activities of novel 1,3-beta-D-glucan synthase inhibitors. Part 2. Bioorg Med Chem Lett11:1273-6 (2001) [PubMed]
Masubuchi, K; Okada, T; Kohchi, M; Murata, T; Tsukazaki, M; Kondoh, O; Yamazaki, T; Satoh, Y; Ono, Y; Tsukaguchi, T; Kobayashi, K; Ono, N; Inoue, T; Horii, I; Shimma, N Synthesis and antifungal activities of novel 1,3-beta-D-glucan synthase inhibitors. Part 2. Bioorg Med Chem Lett11:1273-6 (2001) [PubMed]