| Reaction Details |
|---|
 | Report a problem with these data |
| Target | C-X-C chemokine receptor type 4 |
|---|
| Ligand | BDBM50096735 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_101651 (CHEMBL710307) |
|---|
| IC50 | 10±n/a nM |
|---|
| Citation |  Tamamura, H; Omagari, A; Hiramatsu, K; Gotoh, K; Kanamoto, T; Xu, Y; Kodama, E; Matsuoka, M; Hattori, T; Yamamoto, N; Nakashima, H; Otaka, A; Fujii, N Development of specific CXCR4 inhibitors possessing high selectivity indexes as well as complete stability in serum based on an anti-HIV peptide T140. Bioorg Med Chem Lett11:1897-902 (2001) [PubMed] Tamamura, H; Omagari, A; Hiramatsu, K; Gotoh, K; Kanamoto, T; Xu, Y; Kodama, E; Matsuoka, M; Hattori, T; Yamamoto, N; Nakashima, H; Otaka, A; Fujii, N Development of specific CXCR4 inhibitors possessing high selectivity indexes as well as complete stability in serum based on an anti-HIV peptide T140. Bioorg Med Chem Lett11:1897-902 (2001) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| C-X-C chemokine receptor type 4 |
|---|
| Name: | C-X-C chemokine receptor type 4 |
|---|
| Synonyms: | C-X-C chemokine receptor type 4 | C-X-C chemokine receptor type 4 (CXCR4) | CD_antigen=CD184 | CXC-R4 | CXCR-4 | CXCR4 | CXCR4_HUMAN | FB22 | Fusin | HM89 | LCR1 | LESTR | Leukocyte-derived seven transmembrane domain receptor | NPYRL | SDF-1 receptor | Stromal cell-derived factor 1 receptor |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 39754.61 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P61073 |
|---|
| Residue: | 352 |
|---|
| Sequence: | MEGISIYTSDNYTEEMGSGDYDSMKEPCFREENANFNKIFLPTIYSIIFLTGIVGNGLVI
LVMGYQKKLRSMTDKYRLHLSVADLLFVITLPFWAVDAVANWYFGNFLCKAVHVIYTVNL
YSSVLILAFISLDRYLAIVHATNSQRPRKLLAEKVVYVGVWIPALLLTIPDFIFANVSEA
DDRYICDRFYPNDLWVVVFQFQHIMVGLILPGIVILSCYCIIISKLSHSKGHQKRKALKT
TVILILAFFACWLPYYIGISIDSFILLEIIKQGCEFENTVHKWISITEALAFFHCCLNPI
LYAFLGAKFKTSAQHALTSVSRGSSLKILSKGKRGGHSSVSTESESSSFHSS
|
|
|
|---|
| BDBM50096735 |
|---|
| n/a |
|---|
| Name | BDBM50096735 |
|---|
| Synonyms: | CHEMBL2372983 | Compound T140 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C89H139N33O18S2 |
|---|
| Mol. Mass. | 2023.396 |
|---|
| SMILES | [H][C@]12[#6]-[#6]-[#6]-[#7]1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H](-[#6]-[#16]-[#16]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6]2=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-c1ccc2ccccc2c1 |
|---|
| Structure | 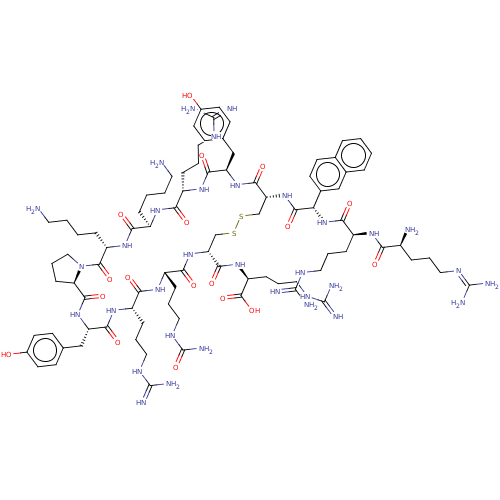
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Tamamura, H; Omagari, A; Hiramatsu, K; Gotoh, K; Kanamoto, T; Xu, Y; Kodama, E; Matsuoka, M; Hattori, T; Yamamoto, N; Nakashima, H; Otaka, A; Fujii, N Development of specific CXCR4 inhibitors possessing high selectivity indexes as well as complete stability in serum based on an anti-HIV peptide T140. Bioorg Med Chem Lett11:1897-902 (2001) [PubMed]
Tamamura, H; Omagari, A; Hiramatsu, K; Gotoh, K; Kanamoto, T; Xu, Y; Kodama, E; Matsuoka, M; Hattori, T; Yamamoto, N; Nakashima, H; Otaka, A; Fujii, N Development of specific CXCR4 inhibitors possessing high selectivity indexes as well as complete stability in serum based on an anti-HIV peptide T140. Bioorg Med Chem Lett11:1897-902 (2001) [PubMed]