| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Reverse transcriptase/RNaseH |
|---|
| Ligand | BDBM50097571 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_195511 |
|---|
| IC50 | 2341±n/a nM |
|---|
| Citation |  Markwalder, JA; Christ, DD; Mutlib, A; Cordova, BC; Klabe, RM; Seitz, SP Synthesis and biological activities of potential metabolites of the non-nucleoside reverse transcriptase inhibitor efavirenz. Bioorg Med Chem Lett11:619-22 (2001) [PubMed] Markwalder, JA; Christ, DD; Mutlib, A; Cordova, BC; Klabe, RM; Seitz, SP Synthesis and biological activities of potential metabolites of the non-nucleoside reverse transcriptase inhibitor efavirenz. Bioorg Med Chem Lett11:619-22 (2001) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Reverse transcriptase/RNaseH |
|---|
| Name: | Reverse transcriptase/RNaseH |
|---|
| Synonyms: | HIV-1 Reverse Transcriptase RNase H | Human immunodeficiency virus type 1 reverse transcriptase | Reverse transcriptase/RNaseH |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 65229.15 |
|---|
| Organism: | Human immunodeficiency virus 1 |
|---|
| Description: | ChEMBL_1473730 |
|---|
| Residue: | 566 |
|---|
| Sequence: | PISPIETVPVKLKPGMDGPKVKQWPLTEEKIKALVEICTEMEKEGKISKIGPENPYNTPV
FAIKKKDSTKWRKLVDFRELNKRTQDFWEVQLGIPHPAGLKKRKSVTVLDVGDAYFSVPL
DEDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFRKQNPDIVI
YQYMDDLYVGSDLEIGQHRTKIEELRQHLLRWGLTTPDKKHQKEPPFLWMGYELHPDKWT
VQPIVLPEKDSWTVNDIQKLVGKLNWASQIYPGIRVRQLCKLLRGTKALTEVIPLTEEAE
LELAENREILKEPVHGVYYDPSKDLIAEIQKQGQGQWTYQIYQEPFKNLRTGKYARMRGA
HTNDVKQLTEAVQKITTESIVIWGKTPKFKLPIQKETWETWWTEYWQATWIPEWEFVNTP
PLVKLWYQLEKEPIVGAETFYVDGAANRETKLGKAGYVTNRGRQKVVTLTDTTNQKTELQ
AIYLALQDSGLEVNIVTDSQYALGIIQAQPDQSESELVNQIIEQLIKKEKVYLAWVPAHK
GIGGNEQVDKLVSAGIRKVLFLDGID
|
|
|
|---|
| BDBM50097571 |
|---|
| n/a |
|---|
| Name | BDBM50097571 |
|---|
| Synonyms: | (S)-6-Chloro-4-cyclopropylethynyl-8-hydroxy-4-trifluoromethyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one | CHEMBL1309 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C14H9ClF3NO3 |
|---|
| Mol. Mass. | 331.674 |
|---|
| SMILES | Oc1cc(Cl)cc2c1NC(=O)O[C@]2(C#CC1CC1)C(F)(F)F |
|---|
| Structure | 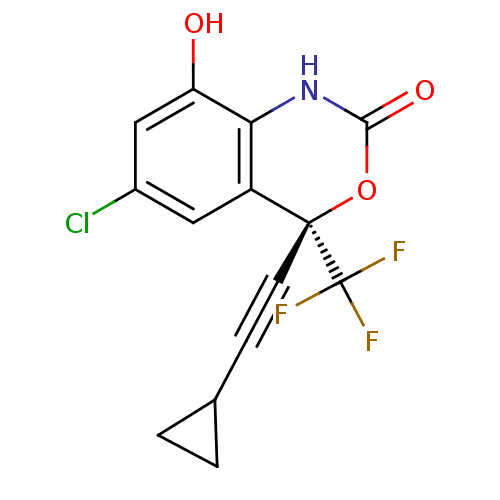
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Markwalder, JA; Christ, DD; Mutlib, A; Cordova, BC; Klabe, RM; Seitz, SP Synthesis and biological activities of potential metabolites of the non-nucleoside reverse transcriptase inhibitor efavirenz. Bioorg Med Chem Lett11:619-22 (2001) [PubMed]
Markwalder, JA; Christ, DD; Mutlib, A; Cordova, BC; Klabe, RM; Seitz, SP Synthesis and biological activities of potential metabolites of the non-nucleoside reverse transcriptase inhibitor efavirenz. Bioorg Med Chem Lett11:619-22 (2001) [PubMed]