| Reaction Details |
|---|
 | Report a problem with these data |
| Target | S-adenosylmethionine synthase isoform type-2 |
|---|
| Ligand | BDBM50452293 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_105118 |
|---|
| Ki | 48000±n/a nM |
|---|
| Citation |  Kappler, F; Hai, TT; Cotter, RJ; Hyver, KJ; Hampton, A Isozyme-specific enzyme inhibitors. 11. L-homocysteine-ATP S-C5' covalent adducts as inhibitors of rat methionine adenosyltransferases. J Med Chem29:1030-8 (1986) [PubMed] Kappler, F; Hai, TT; Cotter, RJ; Hyver, KJ; Hampton, A Isozyme-specific enzyme inhibitors. 11. L-homocysteine-ATP S-C5' covalent adducts as inhibitors of rat methionine adenosyltransferases. J Med Chem29:1030-8 (1986) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| S-adenosylmethionine synthase isoform type-2 |
|---|
| Name: | S-adenosylmethionine synthase isoform type-2 |
|---|
| Synonyms: | AdoMet synthetase 2 | Ams2 | MAT 2 | MAT-II | METK2_RAT | Mat2a | Methionine adenosyltransferase 2 | Methionine adenosyltransferase II | S-Adenosylmethionine Synthase (AdoMet) | S-adenosylmethionine synthetase (MAT 1 and MAT 2) | S-adenosylmethionine synthetase gamma form | S-adenosylmethionine synthetase isoform type-2 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 43713.68 |
|---|
| Organism: | Rattus norvegicus |
|---|
| Description: | ChEMBL_105118 |
|---|
| Residue: | 395 |
|---|
| Sequence: | MNGQLNGFHEAFIEEGTFLFTSESVGEGHPDKICDQINDAVLDAHLQQDPDAKVACETVA
KTGMILLAGEITSRAAIDYQKVVREAIKHIGYDDSSKGFDYKTCNVLVALEQQSPDIAQG
VHLDRNEEDIGAGDQGLMFGYATDETEECMPLTIVLAHKLNAKLAELRRNGTLPWLRPDS
KTQVTVQYMQDRGAVIPIRVHTIVISVQHDEEVCLDEMRDALKEKLIKAVVPAKYLDEDT
IYHLQPSGRFVIGGPQGDAGLTGRKIIVDTYGGWGAHGGGAFSGKDYTKVDRSAAYAARW
VAKSLVKGGLCRRVLVQVSYAIGVSHPLSISIFHYGTSQKSERELLEIVKNNFDLRPGVI
VRDLDLKKPIYQRTAAYGHFGRDSFPWEVPKKLKY
|
|
|
|---|
| BDBM50452293 |
|---|
| n/a |
|---|
| Name | BDBM50452293 |
|---|
| Synonyms: | CHEMBL2092766 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C13H22N5O13P3 |
|---|
| Mol. Mass. | 549.2608 |
|---|
| SMILES | CCC[C@]1(COP(O)(=O)OP(O)(=O)OP(O)(O)=O)O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| |
|---|
| Structure | 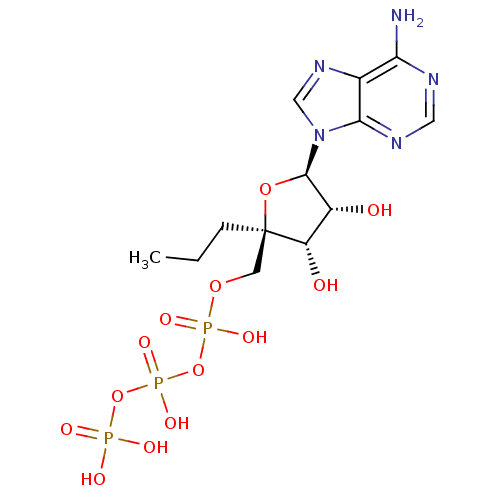
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kappler, F; Hai, TT; Cotter, RJ; Hyver, KJ; Hampton, A Isozyme-specific enzyme inhibitors. 11. L-homocysteine-ATP S-C5' covalent adducts as inhibitors of rat methionine adenosyltransferases. J Med Chem29:1030-8 (1986) [PubMed]
Kappler, F; Hai, TT; Cotter, RJ; Hyver, KJ; Hampton, A Isozyme-specific enzyme inhibitors. 11. L-homocysteine-ATP S-C5' covalent adducts as inhibitors of rat methionine adenosyltransferases. J Med Chem29:1030-8 (1986) [PubMed]