| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Purine nucleoside phosphorylase |
|---|
| Ligand | BDBM50022505 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEBML_153332 |
|---|
| Ki | 30000±n/a nM |
|---|
| Citation |  Sanghvi, YS; Hanna, NB; Larson, SB; Fujitaki, JM; Willis, RC; Smith, RA; Robins, RK; Revankar, GR Synthesis and evaluation of 5-amino-1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamidine and certain related nucleosides as inhibitors of purine nucleoside phosphorylase. J Med Chem31:330-5 (1988) [PubMed] Sanghvi, YS; Hanna, NB; Larson, SB; Fujitaki, JM; Willis, RC; Smith, RA; Robins, RK; Revankar, GR Synthesis and evaluation of 5-amino-1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamidine and certain related nucleosides as inhibitors of purine nucleoside phosphorylase. J Med Chem31:330-5 (1988) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Purine nucleoside phosphorylase |
|---|
| Name: | Purine nucleoside phosphorylase |
|---|
| Synonyms: | Inosine phosphorylase | Inosine-guanosine phosphorylase | NP | PNP | PNPH_HUMAN | Purine nucleoside phosphorylase (PNPase) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 32119.53 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 289 |
|---|
| Sequence: | MENGYTYEDYKNTAEWLLSHTKHRPQVAIICGSGLGGLTDKLTQAQIFDYGEIPNFPRST
VPGHAGRLVFGFLNGRACVMMQGRFHMYEGYPLWKVTFPVRVFHLLGVDTLVVTNAAGGL
NPKFEVGDIMLIRDHINLPGFSGQNPLRGPNDERFGDRFPAMSDAYDRTMRQRALSTWKQ
MGEQRELQEGTYVMVAGPSFETVAECRVLQKLGADAVGMSTVPEVIVARHCGLRVFGFSL
ITNKVIMDYESLEKANHEEVLAAGKQAAQKLEQFVSILMASIPLPDKAS
|
|
|
|---|
| BDBM50022505 |
|---|
| n/a |
|---|
| Name | BDBM50022505 |
|---|
| Synonyms: | 1-(3,4-Dihydroxy-5-hydroxymethyl-tetrahydro-furan-2-yl)-1H-[1,2,4]triazole-3-carboxamidine; hydrochloride | CHEMBL542931 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C8H13N5O4 |
|---|
| Mol. Mass. | 243.2199 |
|---|
| SMILES | NC(=N)c1ncn(n1)C1OC(CO)C(O)[C@@H]1O |
|---|
| Structure | 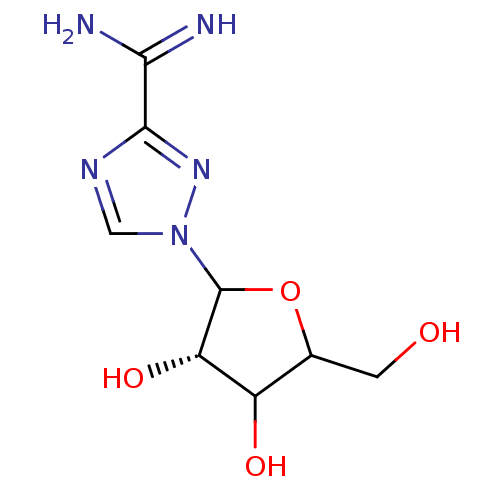
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Sanghvi, YS; Hanna, NB; Larson, SB; Fujitaki, JM; Willis, RC; Smith, RA; Robins, RK; Revankar, GR Synthesis and evaluation of 5-amino-1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamidine and certain related nucleosides as inhibitors of purine nucleoside phosphorylase. J Med Chem31:330-5 (1988) [PubMed]
Sanghvi, YS; Hanna, NB; Larson, SB; Fujitaki, JM; Willis, RC; Smith, RA; Robins, RK; Revankar, GR Synthesis and evaluation of 5-amino-1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamidine and certain related nucleosides as inhibitors of purine nucleoside phosphorylase. J Med Chem31:330-5 (1988) [PubMed]