| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Lactoylglutathione lyase |
|---|
| Ligand | BDBM50039111 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_71294 (CHEMBL684998) |
|---|
| Ki | 1200±n/a nM |
|---|
| Citation |  Murthy, NS; Bakeris, T; Kavarana, MJ; Hamilton, DS; Lan, Y; Creighton, DJ S-(N-aryl-N-hydroxycarbamoyl)glutathione derivatives are tight-binding inhibitors of glyoxalase I and slow substrates for glyoxalase II. J Med Chem37:2161-6 (1994) [PubMed] Murthy, NS; Bakeris, T; Kavarana, MJ; Hamilton, DS; Lan, Y; Creighton, DJ S-(N-aryl-N-hydroxycarbamoyl)glutathione derivatives are tight-binding inhibitors of glyoxalase I and slow substrates for glyoxalase II. J Med Chem37:2161-6 (1994) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Lactoylglutathione lyase |
|---|
| Name: | Lactoylglutathione lyase |
|---|
| Synonyms: | Aldoketomutase | GLO1 | Glx I | Glyoxalase 1 (GLO1) | Glyoxalase I | Ketone-aldehyde mutase | LGUL_HUMAN | Methylglyoxalase | S-D-lactoylglutathione methylglyoxal lyase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 20772.95 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q04760 |
|---|
| Residue: | 184 |
|---|
| Sequence: | MAEPQPPSGGLTDEAALSCCSDADPSTKDFLLQQTMLRVKDPKKSLDFYTRVLGMTLIQK
CDFPIMKFSLYFLAYEDKNDIPKEKDEKIAWALSRKATLELTHNWGTEDDETQSYHNGNS
DPRGFGHIGIAVPDVYSACKRFEELGVKFVKKPDDGKMKGLAFIQDPDGYWIEILNPNKM
ATLM
|
|
|
|---|
| BDBM50039111 |
|---|
| n/a |
|---|
| Name | BDBM50039111 |
|---|
| Synonyms: | S-(N-Hydroxy-N-(4-bromophenyl)carbamoyl)glutathione |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H20BrN4O8S |
|---|
| Mol. Mass. | 520.332 |
|---|
| SMILES | [NH3+]C(CCC(=O)NC(CSC(=O)N(O)c1ccc(Br)cc1)C(=O)NCC([O-])=O)C([O-])=O |
|---|
| Structure | 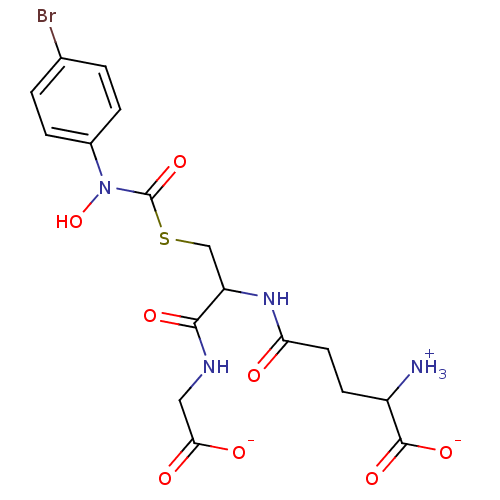
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Murthy, NS; Bakeris, T; Kavarana, MJ; Hamilton, DS; Lan, Y; Creighton, DJ S-(N-aryl-N-hydroxycarbamoyl)glutathione derivatives are tight-binding inhibitors of glyoxalase I and slow substrates for glyoxalase II. J Med Chem37:2161-6 (1994) [PubMed]
Murthy, NS; Bakeris, T; Kavarana, MJ; Hamilton, DS; Lan, Y; Creighton, DJ S-(N-aryl-N-hydroxycarbamoyl)glutathione derivatives are tight-binding inhibitors of glyoxalase I and slow substrates for glyoxalase II. J Med Chem37:2161-6 (1994) [PubMed]