| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Adenosine receptor A3 |
|---|
| Ligand | BDBM50088421 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_30614 (CHEMBL642021) |
|---|
| Ki | 1.4±n/a nM |
|---|
| Citation |  Kim, HO; Ji, XD; Siddiqi, SM; Olah, ME; Stiles, GL; Jacobson, KA 2-Substitution of N6-benzyladenosine-5'-uronamides enhances selectivity for A3 adenosine receptors. J Med Chem37:3614-21 (1994) [PubMed] Kim, HO; Ji, XD; Siddiqi, SM; Olah, ME; Stiles, GL; Jacobson, KA 2-Substitution of N6-benzyladenosine-5'-uronamides enhances selectivity for A3 adenosine receptors. J Med Chem37:3614-21 (1994) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Adenosine receptor A3 |
|---|
| Name: | Adenosine receptor A3 |
|---|
| Synonyms: | AA3R_RAT | Adenosine A3 receptor | Adenosine receptor A2a and A3 | Adora3 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 36643.73 |
|---|
| Organism: | Rattus norvegicus |
|---|
| Description: | ChEMBL_479910 |
|---|
| Residue: | 320 |
|---|
| Sequence: | MKANNTTTSALWLQITYITMEAAIGLCAVVGNMLVIWVVKLNRTLRTTTFYFIVSLALAD
IAVGVLVIPLAIAVSLEVQMHFYACLFMSCVLLVFTHASIMSLLAIAVDRYLRVKLTVRY
RTVTTQRRIWLFLGLCWLVSFLVGLTPMFGWNRKVTLELSQNSSTLSCHFRSVVGLDYMV
FFSFITWILIPLVVMCIIYLDIFYIIRNKLSQNLTGFRETRAFYGREFKTAKSLFLVLFL
FALCWLPLSIINFVSYFNVKIPEIAMCLGILLSHANSMMNPIVYACKIKKFKETYFVILR
ACRLCQTSDSLDSNLEQTTE
|
|
|
|---|
| BDBM50088421 |
|---|
| n/a |
|---|
| Name | BDBM50088421 |
|---|
| Synonyms: | (2R,3R,4S,5R)-2-[2-Chloro-6-(3-iodo-benzylamino)-purin-9-yl]-5-hydroxymethyl-tetrahydro-furan-3,4-diol | 2-[2-Chloro-6-(3-iodo-benzylamino)-purin-9-yl]-5-hydroxymethyl-tetrahydro-furan-3,4-diol | CHEMBL307199 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C17H17ClIN5O4 |
|---|
| Mol. Mass. | 517.705 |
|---|
| SMILES | OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 |
|---|
| Structure | 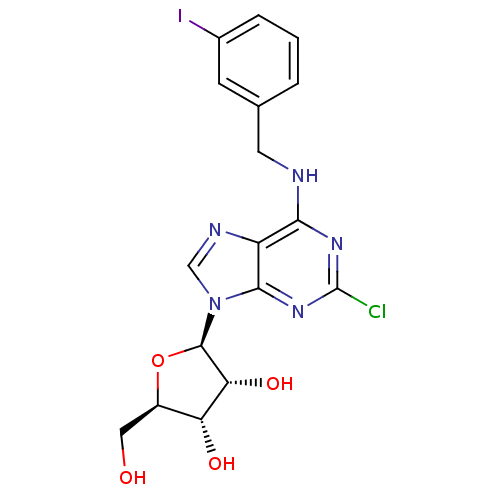
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Kim, HO; Ji, XD; Siddiqi, SM; Olah, ME; Stiles, GL; Jacobson, KA 2-Substitution of N6-benzyladenosine-5'-uronamides enhances selectivity for A3 adenosine receptors. J Med Chem37:3614-21 (1994) [PubMed]
Kim, HO; Ji, XD; Siddiqi, SM; Olah, ME; Stiles, GL; Jacobson, KA 2-Substitution of N6-benzyladenosine-5'-uronamides enhances selectivity for A3 adenosine receptors. J Med Chem37:3614-21 (1994) [PubMed]