| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Polyunsaturated fatty acid 5-lipoxygenase |
|---|
| Ligand | BDBM50029778 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_90070 (CHEMBL701321) |
|---|
| IC50 | 60±n/a nM |
|---|
| Citation |  Stewart, AO; Bhatia, PA; Martin, JG; Summers, JB; Rodriques, KE; Martin, MB; Holms, JH; Moore, JL; Craig, RA; Kolasa, T; Ratajczyk, JD; Mazdiyasni, H; Kerdesky, FA; DeNinno, SL; Maki, RG; Bouska, JB; Young, PR; Lanni, C; Bell, RL; Carter, GW; Brooks, CD Structure-activity relationships of N-hydroxyurea 5-lipoxygenase inhibitors. J Med Chem40:1955-68 (1997) [PubMed] Article Stewart, AO; Bhatia, PA; Martin, JG; Summers, JB; Rodriques, KE; Martin, MB; Holms, JH; Moore, JL; Craig, RA; Kolasa, T; Ratajczyk, JD; Mazdiyasni, H; Kerdesky, FA; DeNinno, SL; Maki, RG; Bouska, JB; Young, PR; Lanni, C; Bell, RL; Carter, GW; Brooks, CD Structure-activity relationships of N-hydroxyurea 5-lipoxygenase inhibitors. J Med Chem40:1955-68 (1997) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Polyunsaturated fatty acid 5-lipoxygenase |
|---|
| Name: | Polyunsaturated fatty acid 5-lipoxygenase |
|---|
| Synonyms: | 5-LO | 5-Lipo-oxygenase (5-LOX) | 5-Lipoxygenase (5-LO) | 5-Lipoxygenase (LOX) | 5-Lipoygenase | 5-lipoxygenase/FLAP | ALOX5 | Arachidonate 5-lipoxygenase | LOG5 | LOX5_HUMAN |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 77972.74 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Recombinant protein was purified from E. coli lysate. After ammonium sulfate precipitation and subsequent steps, the supernatant (S100) was used for 5-LO activity assay.
|
|---|
| Residue: | 674 |
|---|
| Sequence: | MPSYTVTVATGSQWFAGTDDYIYLSLVGSAGCSEKHLLDKPFYNDFERGAVDSYDVTVDE
ELGEIQLVRIEKRKYWLNDDWYLKYITLKTPHGDYIEFPCYRWITGDVEVVLRDGRAKLA
RDDQIHILKQHRRKELETRQKQYRWMEWNPGFPLSIDAKCHKDLPRDIQFDSEKGVDFVL
NYSKAMENLFINRFMHMFQSSWNDFADFEKIFVKISNTISERVMNHWQEDLMFGYQFLNG
CNPVLIRRCTELPEKLPVTTEMVECSLERQLSLEQEVQQGNIFIVDFELLDGIDANKTDP
CTLQFLAAPICLLYKNLANKIVPIAIQLNQIPGDENPIFLPSDAKYDWLLAKIWVRSSDF
HVHQTITHLLRTHLVSEVFGIAMYRQLPAVHPIFKLLVAHVRFTIAINTKAREQLICECG
LFDKANATGGGGHVQMVQRAMKDLTYASLCFPEAIKARGMESKEDIPYYFYRDDGLLVWE
AIRTFTAEVVDIYYEGDQVVEEDPELQDFVNDVYVYGMRGRKSSGFPKSVKSREQLSEYL
TVVIFTASAQHAAVNFGQYDWCSWIPNAPPTMRAPPPTAKGVVTIEQIVDTLPDRGRSCW
HLGAVWALSQFQENELFLGMYPEEHFIEKPVKEAMARFRKNLEAIVSVIAERNKKKQLPY
YYLSPDRIPNSVAI
|
|
|
|---|
| BDBM50029778 |
|---|
| n/a |
|---|
| Name | BDBM50029778 |
|---|
| Synonyms: | CHEMBL264717 | N-{3-[5-(4-fluorophenoxy)-2-furyl]prop-2-ynyl}-N-hydroxyurea | N-{3-[5-(4-fluorophenoxy)furan-2-yl]prop-2-ynyl}-N-hydroxyurea |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C14H11FN2O4 |
|---|
| Mol. Mass. | 290.2465 |
|---|
| SMILES | NC(=O)N(O)CC#Cc1ccc(Oc2ccc(F)cc2)o1 |
|---|
| Structure | 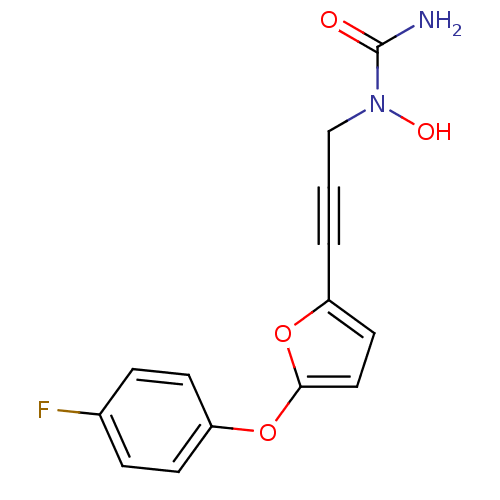
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Stewart, AO; Bhatia, PA; Martin, JG; Summers, JB; Rodriques, KE; Martin, MB; Holms, JH; Moore, JL; Craig, RA; Kolasa, T; Ratajczyk, JD; Mazdiyasni, H; Kerdesky, FA; DeNinno, SL; Maki, RG; Bouska, JB; Young, PR; Lanni, C; Bell, RL; Carter, GW; Brooks, CD Structure-activity relationships of N-hydroxyurea 5-lipoxygenase inhibitors. J Med Chem40:1955-68 (1997) [PubMed] Article
Stewart, AO; Bhatia, PA; Martin, JG; Summers, JB; Rodriques, KE; Martin, MB; Holms, JH; Moore, JL; Craig, RA; Kolasa, T; Ratajczyk, JD; Mazdiyasni, H; Kerdesky, FA; DeNinno, SL; Maki, RG; Bouska, JB; Young, PR; Lanni, C; Bell, RL; Carter, GW; Brooks, CD Structure-activity relationships of N-hydroxyurea 5-lipoxygenase inhibitors. J Med Chem40:1955-68 (1997) [PubMed] Article