| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 1A |
|---|
| Ligand | BDBM50040260 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_1150 (CHEMBL616095) |
|---|
| Ki | 238±n/a nM |
|---|
| Citation |  Chern, JW; Tao, PL; Wang, KC; Gutcait, A; Liu, SW; Yen, MH; Chien, SL; Rong, JK Studies on quinazolines and 1,2,4-benzothiadiazine 1,1-dioxides. 8.1, 2 synthesis and pharmacological evaluation of tricyclic fused quinazolines and 1,2,4-benzothiadiazine 1,1-dioxides as potential alpha1-adrenoceptor antagonists. J Med Chem41:3128-41 (1998) [PubMed] Article Chern, JW; Tao, PL; Wang, KC; Gutcait, A; Liu, SW; Yen, MH; Chien, SL; Rong, JK Studies on quinazolines and 1,2,4-benzothiadiazine 1,1-dioxides. 8.1, 2 synthesis and pharmacological evaluation of tricyclic fused quinazolines and 1,2,4-benzothiadiazine 1,1-dioxides as potential alpha1-adrenoceptor antagonists. J Med Chem41:3128-41 (1998) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 1A |
|---|
| Name: | 5-hydroxytryptamine receptor 1A |
|---|
| Synonyms: | 5-HT-1A | 5-HT1 | 5-HT1A | 5-Hydroxytryptamine receptor 1A (5-HT1A) | 5-hydroxytryptamine receptor 1A (5HT1A) | 5HT1A_RAT | 5ht1a | G-21 | Htr1a | Serotonin 1 (5-HT1) receptor | Serotonin 1a (5-HT1a) receptor/Adrenergic receptor alpha-1 | Serotonin receptor 1A |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 46445.29 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | Binding assays were performed using rat hippocampal membranes. |
|---|
| Residue: | 422 |
|---|
| Sequence: | MDVFSFGQGNNTTASQEPFGTGGNVTSISDVTFSYQVITSLLLGTLIFCAVLGNACVVAA
IALERSLQNVANYLIGSLAVTDLMVSVLVLPMAALYQVLNKWTLGQVTCDLFIALDVLCC
TSSILHLCAIALDRYWAITDPIDYVNKRTPRRAAALISLTWLIGFLISIPPMLGWRTPED
RSDPDACTISKDHGYTIYSTFGAFYIPLLLMLVLYGRIFRAARFRIRKTVRKVEKKGAGT
SLGTSSAPPPKKSLNGQPGSGDWRRCAENRAVGTPCTNGAVRQGDDEATLEVIEVHRVGN
SKEHLPLPSESGSNSYAPACLERKNERNAEAKRKMALARERKTVKTLGIIMGTFILCWLP
FFIVALVLPFCESSCHMPALLGAIINWLGYSNSLLNPVIYAYFNKDFQNAFKKIIKCKFC
RR
|
|
|
|---|
| BDBM50040260 |
|---|
| n/a |
|---|
| Name | BDBM50040260 |
|---|
| Synonyms: | 2-[4-(2-Methoxy-phenyl)-piperazin-1-ylmethyl]-2,6-dihydro-3H-imidazo[1,2-c]quinazolin-5-one | CHEMBL302021 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H25N5O2 |
|---|
| Mol. Mass. | 391.4662 |
|---|
| SMILES | COc1ccccc1N1CCN(CC2CN3C(=N2)c2ccccc2NC3=O)CC1 |c:17| |
|---|
| Structure | 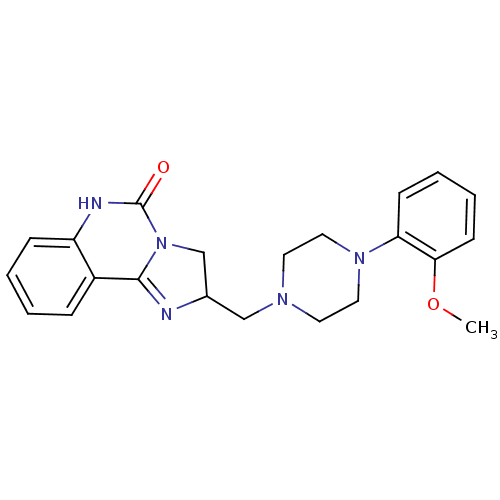
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Chern, JW; Tao, PL; Wang, KC; Gutcait, A; Liu, SW; Yen, MH; Chien, SL; Rong, JK Studies on quinazolines and 1,2,4-benzothiadiazine 1,1-dioxides. 8.1, 2 synthesis and pharmacological evaluation of tricyclic fused quinazolines and 1,2,4-benzothiadiazine 1,1-dioxides as potential alpha1-adrenoceptor antagonists. J Med Chem41:3128-41 (1998) [PubMed] Article
Chern, JW; Tao, PL; Wang, KC; Gutcait, A; Liu, SW; Yen, MH; Chien, SL; Rong, JK Studies on quinazolines and 1,2,4-benzothiadiazine 1,1-dioxides. 8.1, 2 synthesis and pharmacological evaluation of tricyclic fused quinazolines and 1,2,4-benzothiadiazine 1,1-dioxides as potential alpha1-adrenoceptor antagonists. J Med Chem41:3128-41 (1998) [PubMed] Article