| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Bifunctional purine biosynthesis protein ATIC |
|---|
| Ligand | BDBM22579 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_28428 (CHEMBL875660) |
|---|
| IC50 | 25000±n/a nM |
|---|
| Citation |  Wall, M; Shim, JH; Benkovic, SJ A multisubstrate adduct inhibitor of AICAR transformylase. J Med Chem42:3421-4 (1999) [PubMed] Article Wall, M; Shim, JH; Benkovic, SJ A multisubstrate adduct inhibitor of AICAR transformylase. J Med Chem42:3421-4 (1999) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Bifunctional purine biosynthesis protein ATIC |
|---|
| Name: | Bifunctional purine biosynthesis protein ATIC |
|---|
| Synonyms: | 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase | 5-aminoimidazole-4-carboxamide-ribonucleotide transformylase | AICAR Tfase | AICAR transformylase | ATIC | Aminoimidazole carboxamide ribonucleotide transformylase (AICAR Tfase) | Bifunctional purine biosynthesis protein PURH | IMP Cyclohydrolase (IMPCH) | IMP cyclohydrolase | IMP synthetase | Inosinicase | PUR9_HUMAN | PURH | Phosphoribosylaminoimidazolecarboxamide formyltransferase | Thymidylate synthase/GAR transformylase/AICAR transformylase |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 64616.62 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P31939 |
|---|
| Residue: | 592 |
|---|
| Sequence: | MAPGQLALFSVSDKTGLVEFARNLTALGLNLVASGGTAKALRDAGLAVRDVSELTGFPEM
LGGRVKTLHPAVHAGILARNIPEDNADMARLDFNLIRVVACNLYPFVKTVASPGVTVEEA
VEQIDIGGVTLLRAAAKNHARVTVVCEPEDYVVVSTEMQSSESKDTSLETRRQLALKAFT
HTAQYDEAISDYFRKQYSKGVSQMPLRYGMNPHQTPAQLYTLQPKLPITVLNGAPGFINL
CDALNAWQLVKELKEALGIPAAASFKHVSPAGAAVGIPLSEDEAKVCMVYDLYKTLTPIS
AAYARARGADRMSSFGDFVALSDVCDVPTAKIISREVSDGIIAPGYEEEALTILSKKKNG
NYCVLQMDQSYKPDENEVRTLFGLHLSQKRNNGVVDKSLFSNVVTKNKDLPESALRDLIV
ATIAVKYTQSNSVCYAKNGQVIGIGAGQQSRIHCTRLAGDKANYWWLRHHPQVLSMKFKT
GVKRAEISNAIDQYVTGTIGEDEDLIKWKALFEEVPELLTEAEKKEWVEKLTEVSISSDA
FFPFRDNVDRAKRSGVAYIAAPSGSAADKVVIEACDELGIILAHTNLRLFHH
|
|
|
|---|
| BDBM22579 |
|---|
| n/a |
|---|
| Name | BDBM22579 |
|---|
| Synonyms: | AICAR | Aminoimidazole-4-carboxamide ribonucleotide | CHEMBL483849 | ZMP | {[(2R,3S,4R,5R)-5-(5-amino-4-carbamoyl-1H-imidazol-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}phosphonic acid |
|---|
| Type | Nucleoside or nucleotide |
|---|
| Emp. Form. | C9H15N4O8P |
|---|
| Mol. Mass. | 338.2112 |
|---|
| SMILES | NC(=O)c1ncn([C@@H]2O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]2O)c1N |
|---|
| Structure | 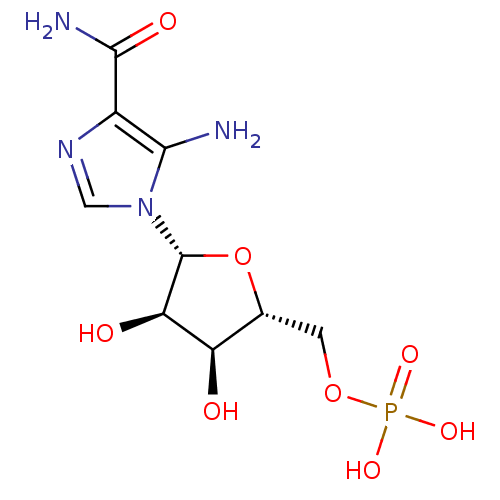
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Wall, M; Shim, JH; Benkovic, SJ A multisubstrate adduct inhibitor of AICAR transformylase. J Med Chem42:3421-4 (1999) [PubMed] Article
Wall, M; Shim, JH; Benkovic, SJ A multisubstrate adduct inhibitor of AICAR transformylase. J Med Chem42:3421-4 (1999) [PubMed] Article