| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Calpain-1 catalytic subunit |
|---|
| Ligand | BDBM50084655 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_43844 (CHEMBL658506) |
|---|
| IC50 | 40±n/a nM |
|---|
| Citation |  Leung, D; Abbenante, G; Fairlie, DP Protease inhibitors: current status and future prospects. J Med Chem43:305-41 (2000) [PubMed] Leung, D; Abbenante, G; Fairlie, DP Protease inhibitors: current status and future prospects. J Med Chem43:305-41 (2000) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Calpain-1 catalytic subunit |
|---|
| Name: | Calpain-1 catalytic subunit |
|---|
| Synonyms: | CAN1_HUMAN | CANPL1 | CAPN1 | Calpain µ-type | Calpain-1 (u-Calpain) | Calpain-1 catalytic subunit | Calpain1 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 81880.51 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 714 |
|---|
| Sequence: | MSEEIITPVYCTGVSAQVQKQRARELGLGRHENAIKYLGQDYEQLRVRCLQSGTLFRDEA
FPPVPQSLGYKDLGPNSSKTYGIKWKRPTELLSNPQFIVDGATRTDICQGALGDCWLLAA
IASLTLNDTLLHRVVPHGQSFQNGYAGIFHFQLWQFGEWVDVVVDDLLPIKDGKLVFVHS
AEGNEFWSALLEKAYAKVNGSYEALSGGSTSEGFEDFTGGVTEWYELRKAPSDLYQIILK
ALERGSLLGCSIDISSVLDMEAITFKKLVKGHAYSVTGAKQVNYRGQVVSLIRMRNPWGE
VEWTGAWSDSSSEWNNVDPYERDQLRVKMEDGEFWMSFRDFMREFTRLEICNLTPDALKS
RTIRKWNTTLYEGTWRRGSTAGGCRNYPATFWVNPQFKIRLDETDDPDDYGDRESGCSFV
LALMQKHRRRERRFGRDMETIGFAVYEVPPELVGQPAVHLKRDFFLANASRARSEQFINL
REVSTRFRLPPGEYVVVPSTFEPNKEGDFVLRFFSEKSAGTVELDDQIQANLPDEQVLSE
EEIDENFKALFRQLAGEDMEISVKELRTILNRIISKHKDLRTKGFSLESCRSMVNLMDRD
GNGKLGLVEFNILWNRIRNYLSIFRKFDLDKSGSMSAYEMRMAIESAGFKLNKKLYELII
TRYSEPDLAVDFDNFVCCLVRLETMFRFFKTLDTDLDGVVTFDLFKWLQLTMFA
|
|
|
|---|
| BDBM50084655 |
|---|
| n/a |
|---|
| Name | BDBM50084655 |
|---|
| Synonyms: | CHEMBL92708 | Calpeptin | Z-Leu-Nle-CHO | [(S)-1-((S)-1-Formyl-pentylcarbamoyl)-3-methyl-butyl]-carbamic acid benzyl ester | [1-((S)-(S)-1-Formyl-pentylcarbamoyl)-3-methyl-butyl]-carbamic acid benzyl ester | [1-(1-Formyl-pentylcarbamoyl)-3-methyl-butyl]-carbamic acid benzyl ester | [1-(1-Formyl-pentylcarbamoyl)-3-methyl-butyl]-carbamic acid benzyl ester(calpeptin) | benzyl (S)-4-methyl-1-oxo-1-((S)-1-oxohexan-2-ylamino)pentan-2-ylcarbamate |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H30N2O4 |
|---|
| Mol. Mass. | 362.4632 |
|---|
| SMILES | CCCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O |r| |
|---|
| Structure | 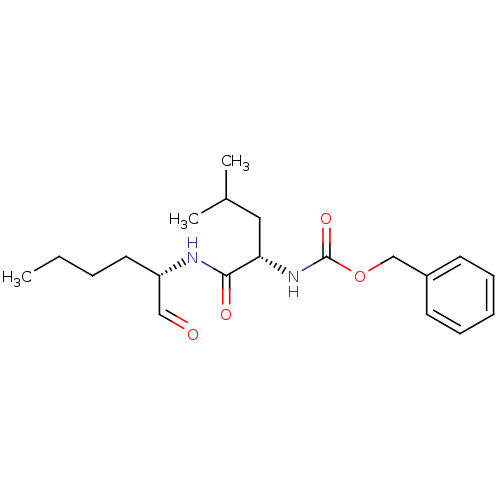
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Leung, D; Abbenante, G; Fairlie, DP Protease inhibitors: current status and future prospects. J Med Chem43:305-41 (2000) [PubMed]
Leung, D; Abbenante, G; Fairlie, DP Protease inhibitors: current status and future prospects. J Med Chem43:305-41 (2000) [PubMed]