| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Reverse transcriptase/RNaseH |
|---|
| Ligand | BDBM50102271 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_196186 (CHEMBL801173) |
|---|
| Ki | 95±n/a nM |
|---|
| Citation |  Campiani, G; Aiello, F; Fabbrini, M; Morelli, E; Ramunno, A; Armaroli, S; Nacci, V; Garofalo, A; Greco, G; Novellino, E; Maga, G; Spadari, S; Bergamini, A; Ventura, L; Bongiovanni, B; Capozzi, M; Bolacchi, F; Marini, S; Coletta, M; Guiso, G; Caccia, S Quinoxalinylethylpyridylthioureas (QXPTs) as potent non-nucleoside HIV-1 reverse transcriptase (RT) inhibitors. Further SAR studies and identification of a novel orally bioavailable hydrazine-based antiviral agent. J Med Chem44:305-15 (2001) [PubMed] Campiani, G; Aiello, F; Fabbrini, M; Morelli, E; Ramunno, A; Armaroli, S; Nacci, V; Garofalo, A; Greco, G; Novellino, E; Maga, G; Spadari, S; Bergamini, A; Ventura, L; Bongiovanni, B; Capozzi, M; Bolacchi, F; Marini, S; Coletta, M; Guiso, G; Caccia, S Quinoxalinylethylpyridylthioureas (QXPTs) as potent non-nucleoside HIV-1 reverse transcriptase (RT) inhibitors. Further SAR studies and identification of a novel orally bioavailable hydrazine-based antiviral agent. J Med Chem44:305-15 (2001) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Reverse transcriptase/RNaseH |
|---|
| Name: | Reverse transcriptase/RNaseH |
|---|
| Synonyms: | HIV-1 Reverse Transcriptase RNase H | Human immunodeficiency virus type 1 reverse transcriptase | Reverse transcriptase/RNaseH |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 65229.15 |
|---|
| Organism: | Human immunodeficiency virus 1 |
|---|
| Description: | ChEMBL_1473730 |
|---|
| Residue: | 566 |
|---|
| Sequence: | PISPIETVPVKLKPGMDGPKVKQWPLTEEKIKALVEICTEMEKEGKISKIGPENPYNTPV
FAIKKKDSTKWRKLVDFRELNKRTQDFWEVQLGIPHPAGLKKRKSVTVLDVGDAYFSVPL
DEDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFRKQNPDIVI
YQYMDDLYVGSDLEIGQHRTKIEELRQHLLRWGLTTPDKKHQKEPPFLWMGYELHPDKWT
VQPIVLPEKDSWTVNDIQKLVGKLNWASQIYPGIRVRQLCKLLRGTKALTEVIPLTEEAE
LELAENREILKEPVHGVYYDPSKDLIAEIQKQGQGQWTYQIYQEPFKNLRTGKYARMRGA
HTNDVKQLTEAVQKITTESIVIWGKTPKFKLPIQKETWETWWTEYWQATWIPEWEFVNTP
PLVKLWYQLEKEPIVGAETFYVDGAANRETKLGKAGYVTNRGRQKVVTLTDTTNQKTELQ
AIYLALQDSGLEVNIVTDSQYALGIIQAQPDQSESELVNQIIEQLIKKEKVYLAWVPAHK
GIGGNEQVDKLVSAGIRKVLFLDGID
|
|
|
|---|
| BDBM50102271 |
|---|
| n/a |
|---|
| Name | BDBM50102271 |
|---|
| Synonyms: | 1-(5-Bromo-pyridin-2-yl)-3-[2-(4-oxo-4H-5,9,9b-triaza-cyclopenta[a]naphthalen-5-yl)-ethyl]-thiourea | CHEMBL107535 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H15BrN6OS |
|---|
| Mol. Mass. | 443.32 |
|---|
| SMILES | Brc1ccc(NC(=S)NCCn2c3cccnc3n3cccc3c2=O)nc1 |
|---|
| Structure | 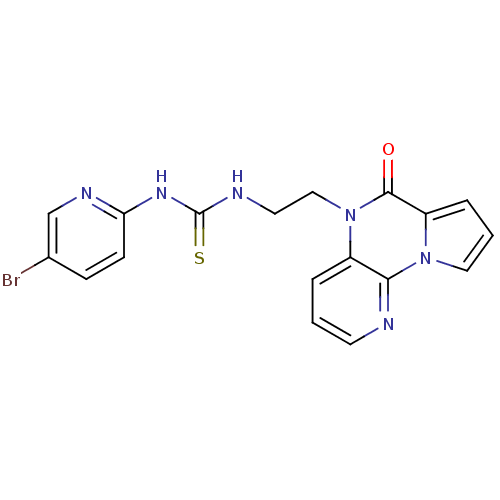
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Campiani, G; Aiello, F; Fabbrini, M; Morelli, E; Ramunno, A; Armaroli, S; Nacci, V; Garofalo, A; Greco, G; Novellino, E; Maga, G; Spadari, S; Bergamini, A; Ventura, L; Bongiovanni, B; Capozzi, M; Bolacchi, F; Marini, S; Coletta, M; Guiso, G; Caccia, S Quinoxalinylethylpyridylthioureas (QXPTs) as potent non-nucleoside HIV-1 reverse transcriptase (RT) inhibitors. Further SAR studies and identification of a novel orally bioavailable hydrazine-based antiviral agent. J Med Chem44:305-15 (2001) [PubMed]
Campiani, G; Aiello, F; Fabbrini, M; Morelli, E; Ramunno, A; Armaroli, S; Nacci, V; Garofalo, A; Greco, G; Novellino, E; Maga, G; Spadari, S; Bergamini, A; Ventura, L; Bongiovanni, B; Capozzi, M; Bolacchi, F; Marini, S; Coletta, M; Guiso, G; Caccia, S Quinoxalinylethylpyridylthioureas (QXPTs) as potent non-nucleoside HIV-1 reverse transcriptase (RT) inhibitors. Further SAR studies and identification of a novel orally bioavailable hydrazine-based antiviral agent. J Med Chem44:305-15 (2001) [PubMed]