| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Urokinase-type plasminogen activator |
|---|
| Ligand | BDBM12657 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_212982 (CHEMBL817003) |
|---|
| Ki | >19000±n/a nM |
|---|
| Citation |  Pinto, DJ; Orwat, MJ; Wang, S; Fevig, JM; Quan, ML; Amparo, E; Cacciola, J; Rossi, KA; Alexander, RS; Smallwood, AM; Luettgen, JM; Liang, L; Aungst, BJ; Wright, MR; Knabb, RM; Wong, PC; Wexler, RR; Lam, PY Discovery of 1-[3-(aminomethyl)phenyl]-N-3-fluoro-2'-(methylsulfonyl)-[1,1'-biphenyl]-4-yl]-3-(trifluoromethyl)-1H-pyrazole-5-carboxamide (DPC423), a highly potent, selective, and orally bioavailable inhibitor of blood coagulation factor Xa. J Med Chem44:566-78 (2001) [PubMed] Pinto, DJ; Orwat, MJ; Wang, S; Fevig, JM; Quan, ML; Amparo, E; Cacciola, J; Rossi, KA; Alexander, RS; Smallwood, AM; Luettgen, JM; Liang, L; Aungst, BJ; Wright, MR; Knabb, RM; Wong, PC; Wexler, RR; Lam, PY Discovery of 1-[3-(aminomethyl)phenyl]-N-3-fluoro-2'-(methylsulfonyl)-[1,1'-biphenyl]-4-yl]-3-(trifluoromethyl)-1H-pyrazole-5-carboxamide (DPC423), a highly potent, selective, and orally bioavailable inhibitor of blood coagulation factor Xa. J Med Chem44:566-78 (2001) [PubMed] |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Urokinase-type plasminogen activator |
|---|
| Name: | Urokinase-type plasminogen activator |
|---|
| Synonyms: | PLAU | U-plasminogen activator | UROK_HUMAN | Urokinase | Urokinase-type plasminogen activator (uPA) | Urokinase-type plasminogen activator/surface receptor |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 48528.62 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P00749 |
|---|
| Residue: | 431 |
|---|
| Sequence: | MRALLARLLLCVLVVSDSKGSNELHQVPSNCDCLNGGTCVSNKYFSNIHWCNCPKKFGGQ
HCEIDKSKTCYEGNGHFYRGKASTDTMGRPCLPWNSATVLQQTYHAHRSDALQLGLGKHN
YCRNPDNRRRPWCYVQVGLKPLVQECMVHDCADGKKPSSPPEELKFQCGQKTLRPRFKII
GGEFTTIENQPWFAAIYRRHRGGSVTYVCGGSLISPCWVISATHCFIDYPKKEDYIVYLG
RSRLNSNTQGEMKFEVENLILHKDYSADTLAHHNDIALLKIRSKEGRCAQPSRTIQTICL
PSMYNDPQFGTSCEITGFGKENSTDYLYPEQLKMTVVKLISHRECQQPHYYGSEVTTKML
CAADPQWKTDSCQGDSGGPLVCSLQGRMTLTGIVSWGRGCALKDKPGVYTRVSHFLPWIR
SHTKEENGLAL
|
|
|
|---|
| BDBM12657 |
|---|
| n/a |
|---|
| Name | BDBM12657 |
|---|
| Synonyms: | 1-[3-(Aminomethyl)phenyl]-N-[3-fluoro-2-(methylsulfonyl)-[1,1-biphenyl]-4-yl]-3-(trifluoromethyl)-1H-pyrazole-5-carboxamide | 1-[3-(aminomethyl)phenyl]-N-[2-fluoro-4-(2-methanesulfonylphenyl)phenyl]-3-(trifluoromethyl)-1H-pyrazole-5-carboxamide | CHEMBL559015 | DPC 423 | DPC423 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H20F4N4O3S |
|---|
| Mol. Mass. | 532.51 |
|---|
| SMILES | CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)c2cc(nn2-c2cccc(CN)c2)C(F)(F)F)c(F)c1 |
|---|
| Structure | 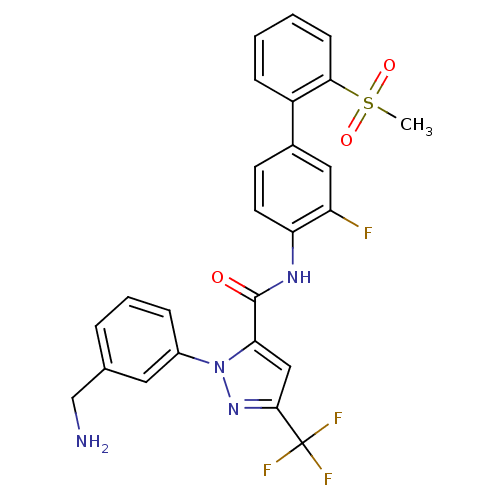
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Pinto, DJ; Orwat, MJ; Wang, S; Fevig, JM; Quan, ML; Amparo, E; Cacciola, J; Rossi, KA; Alexander, RS; Smallwood, AM; Luettgen, JM; Liang, L; Aungst, BJ; Wright, MR; Knabb, RM; Wong, PC; Wexler, RR; Lam, PY Discovery of 1-[3-(aminomethyl)phenyl]-N-3-fluoro-2'-(methylsulfonyl)-[1,1'-biphenyl]-4-yl]-3-(trifluoromethyl)-1H-pyrazole-5-carboxamide (DPC423), a highly potent, selective, and orally bioavailable inhibitor of blood coagulation factor Xa. J Med Chem44:566-78 (2001) [PubMed]
Pinto, DJ; Orwat, MJ; Wang, S; Fevig, JM; Quan, ML; Amparo, E; Cacciola, J; Rossi, KA; Alexander, RS; Smallwood, AM; Luettgen, JM; Liang, L; Aungst, BJ; Wright, MR; Knabb, RM; Wong, PC; Wexler, RR; Lam, PY Discovery of 1-[3-(aminomethyl)phenyl]-N-3-fluoro-2'-(methylsulfonyl)-[1,1'-biphenyl]-4-yl]-3-(trifluoromethyl)-1H-pyrazole-5-carboxamide (DPC423), a highly potent, selective, and orally bioavailable inhibitor of blood coagulation factor Xa. J Med Chem44:566-78 (2001) [PubMed]