| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Collagenase 3 |
|---|
| Ligand | BDBM50128642 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_106632 (CHEMBL717016) |
|---|
| IC50 | 3±n/a nM |
|---|
| Citation |  Aranapakam, V; Davis, JM; Grosu, GT; Baker, J; Ellingboe, J; Zask, A; Levin, JI; Sandanayaka, VP; Du, M; Skotnicki, JS; DiJoseph, JF; Sung, A; Sharr, MA; Killar, LM; Walter, T; Jin, G; Cowling, R; Tillett, J; Zhao, W; McDevitt, J; Xu, ZB Synthesis and structure-activity relationship of N-substituted 4-arylsulfonylpiperidine-4-hydroxamic acids as novel, orally active matrix metalloproteinase inhibitors for the treatment of osteoarthritis. J Med Chem46:2376-96 (2003) [PubMed] Article Aranapakam, V; Davis, JM; Grosu, GT; Baker, J; Ellingboe, J; Zask, A; Levin, JI; Sandanayaka, VP; Du, M; Skotnicki, JS; DiJoseph, JF; Sung, A; Sharr, MA; Killar, LM; Walter, T; Jin, G; Cowling, R; Tillett, J; Zhao, W; McDevitt, J; Xu, ZB Synthesis and structure-activity relationship of N-substituted 4-arylsulfonylpiperidine-4-hydroxamic acids as novel, orally active matrix metalloproteinase inhibitors for the treatment of osteoarthritis. J Med Chem46:2376-96 (2003) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Collagenase 3 |
|---|
| Name: | Collagenase 3 |
|---|
| Synonyms: | MMP-13 | MMP13 | MMP13_HUMAN | Matrix metalloproteinase-13 | Matrix metalloproteinase-13 (MMP-13) | Matrix metalloproteinase-13 (MMP13) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 53808.06 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P45452 |
|---|
| Residue: | 471 |
|---|
| Sequence: | MHPGVLAAFLFLSWTHCRALPLPSGGDEDDLSEEDLQFAERYLRSYYHPTNLAGILKENA
ASSMTERLREMQSFFGLEVTGKLDDNTLDVMKKPRCGVPDVGEYNVFPRTLKWSKMNLTY
RIVNYTPDMTHSEVEKAFKKAFKVWSDVTPLNFTRLHDGIADIMISFGIKEHGDFYPFDG
PSGLLAHAFPPGPNYGGDAHFDDDETWTSSSKGYNLFLVAAHEFGHSLGLDHSKDPGALM
FPIYTYTGKSHFMLPDDDVQGIQSLYGPGDEDPNPKHPKTPDKCDPSLSLDAITSLRGET
MIFKDRFFWRLHPQQVDAELFLTKSFWPELPNRIDAAYEHPSHDLIFIFRGRKFWALNGY
DILEGYPKKISELGLPKEVKKISAAVHFEDTGKTLLFSGNQVWRYDDTNHIMDKDYPRLI
EEDFPGIGDKVDAVYEKNGYIYFFNGPIQFEYSIWSNRIVRVMPANSILWC
|
|
|
|---|
| BDBM50128642 |
|---|
| n/a |
|---|
| Name | BDBM50128642 |
|---|
| Synonyms: | 1-(4-(thiophen-2-yl)benzyl)-N-hydroxy-4-(4-methoxyphenylsulfonyl)piperidine-4-carboxamide | 4-(4-Methoxy-benzenesulfonyl)-1-(4-thiophen-2-yl-benzyl)-piperidine-4-carboxylic acid hydroxyamide | CHEMBL80183 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H26N2O5S2 |
|---|
| Mol. Mass. | 486.604 |
|---|
| SMILES | COc1ccc(cc1)S(=O)(=O)C1(CCN(Cc2ccc(cc2)-c2cccs2)CC1)C(=O)NO |
|---|
| Structure | 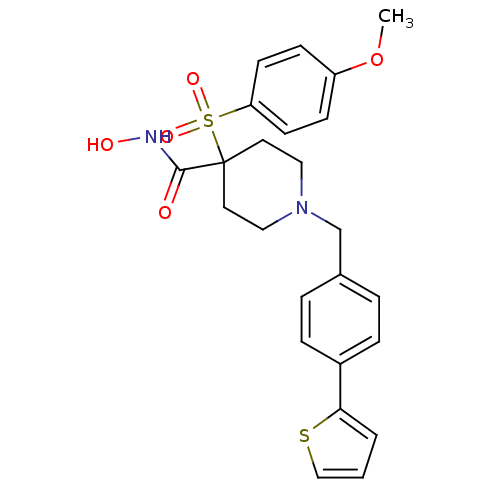
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Aranapakam, V; Davis, JM; Grosu, GT; Baker, J; Ellingboe, J; Zask, A; Levin, JI; Sandanayaka, VP; Du, M; Skotnicki, JS; DiJoseph, JF; Sung, A; Sharr, MA; Killar, LM; Walter, T; Jin, G; Cowling, R; Tillett, J; Zhao, W; McDevitt, J; Xu, ZB Synthesis and structure-activity relationship of N-substituted 4-arylsulfonylpiperidine-4-hydroxamic acids as novel, orally active matrix metalloproteinase inhibitors for the treatment of osteoarthritis. J Med Chem46:2376-96 (2003) [PubMed] Article
Aranapakam, V; Davis, JM; Grosu, GT; Baker, J; Ellingboe, J; Zask, A; Levin, JI; Sandanayaka, VP; Du, M; Skotnicki, JS; DiJoseph, JF; Sung, A; Sharr, MA; Killar, LM; Walter, T; Jin, G; Cowling, R; Tillett, J; Zhao, W; McDevitt, J; Xu, ZB Synthesis and structure-activity relationship of N-substituted 4-arylsulfonylpiperidine-4-hydroxamic acids as novel, orally active matrix metalloproteinase inhibitors for the treatment of osteoarthritis. J Med Chem46:2376-96 (2003) [PubMed] Article