| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Lanosterol synthase |
|---|
| Ligand | BDBM50128050 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_80 (CHEMBL615201) |
|---|
| IC50 | 5.4±n/a nM |
|---|
| Citation |  Dehmlow, H; Aebi, JD; Jolidon, S; Ji, YH; von der Mark, EM; Himber, J; Morand, OH Synthesis and structure-activity studies of novel orally active non-terpenoic 2,3-oxidosqualene cyclase inhibitors. J Med Chem46:3354-70 (2003) [PubMed] Article Dehmlow, H; Aebi, JD; Jolidon, S; Ji, YH; von der Mark, EM; Himber, J; Morand, OH Synthesis and structure-activity studies of novel orally active non-terpenoic 2,3-oxidosqualene cyclase inhibitors. J Med Chem46:3354-70 (2003) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Lanosterol synthase |
|---|
| Name: | Lanosterol synthase |
|---|
| Synonyms: | 2,3-epoxysqualene--lanosterol cyclase | LSS | LSS_HUMAN | OSC | Oxidosqualene--lanosterol cyclase |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 83309.32 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_11231 |
|---|
| Residue: | 732 |
|---|
| Sequence: | MTEGTCLRRRGGPYKTEPATDLGRWRLNCERGRQTWTYLQDERAGREQTGLEAYALGLDT
KNYFKDLPKAHTAFEGALNGMTFYVGLQAEDGHWTGDYGGPLFLLPGLLITCHVARIPLP
AGYREEIVRYLRSVQLPDGGWGLHIEDKSTVFGTALNYVSLRILGVGPDDPDLVRARNIL
HKKGGAVAIPSWGKFWLAVLNVYSWEGLNTLFPEMWLFPDWAPAHPSTLWCHCRQVYLPM
SYCYAVRLSAAEDPLVQSLRQELYVEDFASIDWLAQRNNVAPDELYTPHSWLLRVVYALL
NLYEHHHSAHLRQRAVQKLYEHIVADDRFTKSISIGPISKTINMLVRWYVDGPASTAFQE
HVSRIPDYLWMGLDGMKMQGTNGSQIWDTAFAIQALLEAGGHHRPEFSSCLQKAHEFLRL
SQVPDNPPDYQKYYRQMRKGGFSFSTLDCGWIVSDCTAEALKAVLLLQEKCPHVTEHIPR
ERLCDAVAVLLNMRNPDGGFATYETKRGGHLLELLNPSEVFGDIMIDYTYVECTSAVMQA
LKYFHKRFPEHRAAEIRETLTQGLEFCRRQQRADGSWEGSWGVCFTYGTWFGLEAFACMG
QTYRDGTACAEVSRACDFLLSRQMADGGWGEDFESCEERRYLQSAQSQIHNTCWAMMGLM
AVRHPDIEAQERGVRCLLEKQLPNGDWPQENIAGVFNKSCAISYTSYRNIFPIWALGRFS
QLYPERALAGHP
|
|
|
|---|
| BDBM50128050 |
|---|
| n/a |
|---|
| Name | BDBM50128050 |
|---|
| Synonyms: | CHEMBL114259 | CHEMBL416694 | N-allyl-6-(4-(4-bromobenzoyl)phenoxy)-N-methylhexan-1-aminium | {4-[6-(Allyl-methyl-amino)-hexyloxy]-phenyl}-(4-bromo-phenyl)-methanone |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H28BrNO2 |
|---|
| Mol. Mass. | 430.378 |
|---|
| SMILES | CN(CCCCCCOc1ccc(cc1)C(=O)c1ccc(Br)cc1)CC=C |
|---|
| Structure | 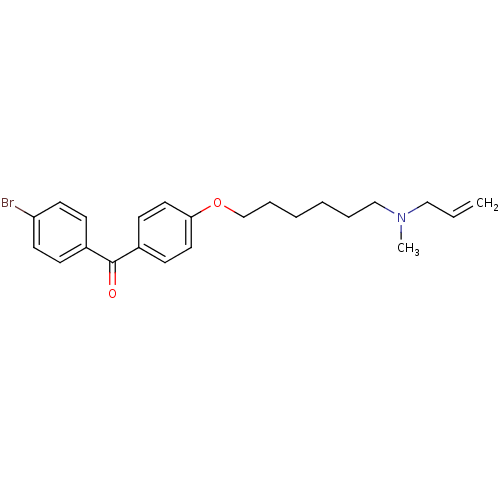
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Dehmlow, H; Aebi, JD; Jolidon, S; Ji, YH; von der Mark, EM; Himber, J; Morand, OH Synthesis and structure-activity studies of novel orally active non-terpenoic 2,3-oxidosqualene cyclase inhibitors. J Med Chem46:3354-70 (2003) [PubMed] Article
Dehmlow, H; Aebi, JD; Jolidon, S; Ji, YH; von der Mark, EM; Himber, J; Morand, OH Synthesis and structure-activity studies of novel orally active non-terpenoic 2,3-oxidosqualene cyclase inhibitors. J Med Chem46:3354-70 (2003) [PubMed] Article