| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Chymotrypsinogen B |
|---|
| Ligand | BDBM50126829 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_49607 (CHEMBL661258) |
|---|
| IC50 | 25000±n/a nM |
|---|
| Citation |  McGovern, SL; Shoichet, BK Kinase inhibitors: not just for kinases anymore. J Med Chem46:1478-83 (2003) [PubMed] Article McGovern, SL; Shoichet, BK Kinase inhibitors: not just for kinases anymore. J Med Chem46:1478-83 (2003) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Chymotrypsinogen B |
|---|
| Name: | Chymotrypsinogen B |
|---|
| Synonyms: | A0A2R8YG87_HUMAN | Beta-chymotrypsin | CTRB1 | Chymotrypsin B chain A | Chymotrypsin B chain B | Chymotrypsin B chain C | Chymotrypsinogen B |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 27871.19 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_216634 |
|---|
| Residue: | 263 |
|---|
| Sequence: | MAFLWLLSCWALLGTTFGCGVPAIHPVLSGLSRIVNGEDAVPGSWPWQVSLQDKTGFHFC
GGSLISEDWVVTAAHCGVRTSDVVVAGEFDQGSDEENIQVLKIAKVFKNPKFSILTVNND
ITLLKLATPARFSQTVSAVCLPSADDDFPAGTLCATTGWGKTKYNANKTPDKLQQAALPL
LSNAECKKSWGRRITDVMICAGASGVSSCMGDSGGPLVCQKDGAWTLVGIVSWGSDTCST
SSPGVYARVTKLIPWVQKILAAN
|
|
|
|---|
| BDBM50126829 |
|---|
| n/a |
|---|
| Name | BDBM50126829 |
|---|
| Synonyms: | (E)-1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl)-5,7-dihydroxy-2,2-dimethyl-2H-chromen-8-yl)-3-phenylprop-2-en-1-one | 1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl)-5,7-dihydroxy-2,2-dimethyl-2H-chromen-8-yl)-3-phenylprop-2-en-1-one | 1-[6-(3-Acetyl-2,4,6-trihydroxy-5-methyl-benzyl)-5,7-dihydroxy-2,2-dimethyl-2H-chromen-8-yl]-3-phenyl-propenone | CHEMBL34241 | R5648 (Rottlerin) | ROTTLERIN |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C30H28O8 |
|---|
| Mol. Mass. | 516.5385 |
|---|
| SMILES | CC(=O)c1c(O)c(C)c(O)c(Cc2c(O)c3C=CC(C)(C)Oc3c(C(=O)C=Cc3ccccc3)c2O)c1O |w:26.26,c:16| |
|---|
| Structure | 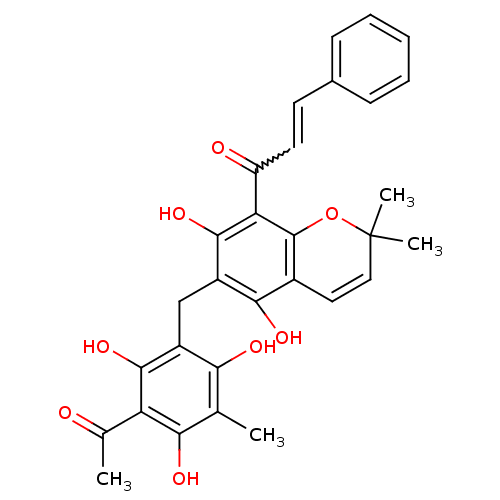
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 McGovern, SL; Shoichet, BK Kinase inhibitors: not just for kinases anymore. J Med Chem46:1478-83 (2003) [PubMed] Article
McGovern, SL; Shoichet, BK Kinase inhibitors: not just for kinases anymore. J Med Chem46:1478-83 (2003) [PubMed] Article