| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Delta-type opioid receptor |
|---|
| Ligand | BDBM50130561 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_146497 (CHEMBL754607) |
|---|
| Ki | >4900±n/a nM |
|---|
| Citation |  Thomas, JB; Fix, SE; Rothman, RB; Mascarella, SW; Dersch, CM; Cantrell, BE; Zimmerman, DM; Carroll, FI Importance of phenolic address groups in opioid kappa receptor selective antagonists. J Med Chem47:1070-3 (2004) [PubMed] Article Thomas, JB; Fix, SE; Rothman, RB; Mascarella, SW; Dersch, CM; Cantrell, BE; Zimmerman, DM; Carroll, FI Importance of phenolic address groups in opioid kappa receptor selective antagonists. J Med Chem47:1070-3 (2004) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Delta-type opioid receptor |
|---|
| Name: | Delta-type opioid receptor |
|---|
| Synonyms: | Cytochrome P450 3A4 | DOR-1 | Delta opioid receptor | Delta-type opioid receptor | Delta-type opioid receptor (DOR) | OPIATE Delta | OPRD_RAT | Opiate Delta 1 | Opioid receptor | Opioid receptor A | Opioid receptors; mu & delta | Oprd1 | Ror-a | Voltage-gated potassium channel |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 40465.04 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | Competition binding assays were using CHO-K1 cell membranes expressing the opioid receptor. |
|---|
| Residue: | 372 |
|---|
| Sequence: | MEPVPSARAELQFSLLANVSDTFPSAFPSASANASGSPGARSASSLALAIAITALYSAVC
AVGLLGNVLVMFGIVRYTKLKTATNIYIFNLALADALATSTLPFQSAKYLMETWPFGELL
CKAVLSIDYYNMFTSIFTLTMMSVDRYIAVCHPVKALDFRTPAKAKLINICIWVLASGVG
VPIMVMAVTQPRDGAVVCTLQFPSPSWYWDTVTKICVFLFAFVVPILIITVCYGLMLLRL
RSVRLLSGSKEKDRSLRRITRMVLVVVGAFVVCWAPIHIFVIVWTLVDINRRDPLVVAAL
HLCIALGYANSSLNPVLYAFLDENFKRCFRQLCRAPCGGQEPGSLRRPRQATARERVTAC
TPSDGPGGGAAA
|
|
|
|---|
| BDBM50130561 |
|---|
| n/a |
|---|
| Name | BDBM50130561 |
|---|
| Synonyms: | (R)-N-((S)-1-((3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl)-3-methylbutan-2-yl)-1,2,3,4-tetrahydroisoquinoline-3-carboxamide | 1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid {(S)-1-[(3R,4R)-4-(3-hydroxy-phenyl)-3,4-dimethyl-piperidin-1-ylmethyl]-2-methyl-propyl}-amide | 1,2,3,4-Tetrahydro-isoquinoline-3-carboxylic acid {1-[4-(3-hydroxy-phenyl)-3,4-dimethyl-piperidin-1-ylmethyl]-2-methyl-propyl}-amide | CHEMBL10872 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C28H39N3O2 |
|---|
| Mol. Mass. | 449.6282 |
|---|
| SMILES | CC(C)[C@@H](CN1CC[C@](C)([C@@H](C)C1)c1cccc(O)c1)NC(=O)[C@H]1Cc2ccccc2CN1 |
|---|
| Structure | 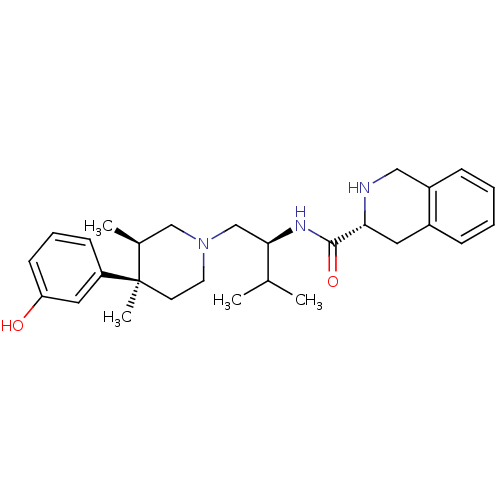
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Thomas, JB; Fix, SE; Rothman, RB; Mascarella, SW; Dersch, CM; Cantrell, BE; Zimmerman, DM; Carroll, FI Importance of phenolic address groups in opioid kappa receptor selective antagonists. J Med Chem47:1070-3 (2004) [PubMed] Article
Thomas, JB; Fix, SE; Rothman, RB; Mascarella, SW; Dersch, CM; Cantrell, BE; Zimmerman, DM; Carroll, FI Importance of phenolic address groups in opioid kappa receptor selective antagonists. J Med Chem47:1070-3 (2004) [PubMed] Article