| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Prothrombin |
|---|
| Ligand | BDBM50133531 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_208532 (CHEMBL813626) |
|---|
| Ki | 250±n/a nM |
|---|
| Citation |  Young, MB; Barrow, JC; Glass, KL; Lundell, GF; Newton, CL; Pellicore, JM; Rittle, KE; Selnick, HG; Stauffer, KJ; Vacca, JP; Williams, PD; Bohn, D; Clayton, FC; Cook, JJ; Krueger, JA; Kuo, LC; Lewis, SD; Lucas, BJ; McMasters, DR; Miller-Stein, C; Pietrak, BL; Wallace, AA; White, RB; Wong, B; Yan, Y; Nantermet, PG Discovery and evaluation of potent P1 aryl heterocycle-based thrombin inhibitors. J Med Chem47:2995-3008 (2004) [PubMed] Article Young, MB; Barrow, JC; Glass, KL; Lundell, GF; Newton, CL; Pellicore, JM; Rittle, KE; Selnick, HG; Stauffer, KJ; Vacca, JP; Williams, PD; Bohn, D; Clayton, FC; Cook, JJ; Krueger, JA; Kuo, LC; Lewis, SD; Lucas, BJ; McMasters, DR; Miller-Stein, C; Pietrak, BL; Wallace, AA; White, RB; Wong, B; Yan, Y; Nantermet, PG Discovery and evaluation of potent P1 aryl heterocycle-based thrombin inhibitors. J Med Chem47:2995-3008 (2004) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Prothrombin |
|---|
| Name: | Prothrombin |
|---|
| Synonyms: | Activation peptide fragment 1 | Activation peptide fragment 2 | Coagulation factor II | F2 | Prothrombin precursor | THRB_HUMAN | Thrombin heavy chain | Thrombin light chain |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 70029.57 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P00734 |
|---|
| Residue: | 622 |
|---|
| Sequence: | MAHVRGLQLPGCLALAALCSLVHSQHVFLAPQQARSLLQRVRRANTFLEEVRKGNLEREC
VEETCSYEEAFEALESSTATDVFWAKYTACETARTPRDKLAACLEGNCAEGLGTNYRGHV
NITRSGIECQLWRSRYPHKPEINSTTHPGADLQENFCRNPDSSTTGPWCYTTDPTVRRQE
CSIPVCGQDQVTVAMTPRSEGSSVNLSPPLEQCVPDRGQQYQGRLAVTTHGLPCLAWASA
QAKALSKHQDFNSAVQLVENFCRNPDGDEEGVWCYVAGKPGDFGYCDLNYCEEAVEEETG
DGLDEDSDRAIEGRTATSEYQTFFNPRTFGSGEADCGLRPLFEKKSLEDKTERELLESYI
DGRIVEGSDAEIGMSPWQVMLFRKSPQELLCGASLISDRWVLTAAHCLLYPPWDKNFTEN
DLLVRIGKHSRTRYERNIEKISMLEKIYIHPRYNWRENLDRDIALMKLKKPVAFSDYIHP
VCLPDRETAASLLQAGYKGRVTGWGNLKETWTANVGKGQPSVLQVVNLPIVERPVCKDST
RIRITDNMFCAGYKPDEGKRGDACEGDSGGPFVMKSPFNNRWYQMGIVSWGEGCDRDGKY
GFYTHVFRLKKWIQKVIDQFGE
|
|
|
|---|
| BDBM50133531 |
|---|
| n/a |
|---|
| Name | BDBM50133531 |
|---|
| Synonyms: | (S)-1-((R)-2-Amino-3-phenyl-propionyl)-pyrrolidine-2-carboxylic acid 3-chloro-benzylamide | 1-((S)-2-Amino-1-(R)-oxo-3-phenyl-propyl)-pyrrolidine-2-carboxylic acid 3-chloro-benzylamide | CHEMBL321130 | D-phenylalanyl-N-(3-chlorobenzyl)-L-prolinamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H24ClN3O2 |
|---|
| Mol. Mass. | 385.887 |
|---|
| SMILES | N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1cccc(Cl)c1 |
|---|
| Structure | 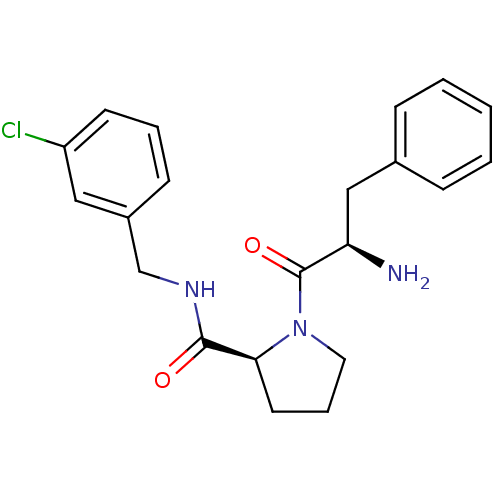
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Young, MB; Barrow, JC; Glass, KL; Lundell, GF; Newton, CL; Pellicore, JM; Rittle, KE; Selnick, HG; Stauffer, KJ; Vacca, JP; Williams, PD; Bohn, D; Clayton, FC; Cook, JJ; Krueger, JA; Kuo, LC; Lewis, SD; Lucas, BJ; McMasters, DR; Miller-Stein, C; Pietrak, BL; Wallace, AA; White, RB; Wong, B; Yan, Y; Nantermet, PG Discovery and evaluation of potent P1 aryl heterocycle-based thrombin inhibitors. J Med Chem47:2995-3008 (2004) [PubMed] Article
Young, MB; Barrow, JC; Glass, KL; Lundell, GF; Newton, CL; Pellicore, JM; Rittle, KE; Selnick, HG; Stauffer, KJ; Vacca, JP; Williams, PD; Bohn, D; Clayton, FC; Cook, JJ; Krueger, JA; Kuo, LC; Lewis, SD; Lucas, BJ; McMasters, DR; Miller-Stein, C; Pietrak, BL; Wallace, AA; White, RB; Wong, B; Yan, Y; Nantermet, PG Discovery and evaluation of potent P1 aryl heterocycle-based thrombin inhibitors. J Med Chem47:2995-3008 (2004) [PubMed] Article