| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Thymidine phosphorylase |
|---|
| Ligand | BDBM50122764 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_305155 (CHEMBL832607) |
|---|
| IC50 | 19±n/a nM |
|---|
| Citation |  Reigan, P; Edwards, PN; Gbaj, A; Cole, C; Barry, ST; Page, KM; Ashton, SE; Luke, RW; Douglas, KT; Stratford, IJ; Jaffar, M; Bryce, RA; Freeman, S Aminoimidazolylmethyluracil analogues as potent inhibitors of thymidine phosphorylase and their bioreductive nitroimidazolyl prodrugs. J Med Chem48:392-402 (2005) [PubMed] Article Reigan, P; Edwards, PN; Gbaj, A; Cole, C; Barry, ST; Page, KM; Ashton, SE; Luke, RW; Douglas, KT; Stratford, IJ; Jaffar, M; Bryce, RA; Freeman, S Aminoimidazolylmethyluracil analogues as potent inhibitors of thymidine phosphorylase and their bioreductive nitroimidazolyl prodrugs. J Med Chem48:392-402 (2005) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Thymidine phosphorylase |
|---|
| Name: | Thymidine phosphorylase |
|---|
| Synonyms: | ECGF1 | Gliostatin | PD-ECGF | Platelet-derived endothelial cell growth factor | TP | TYMP | TYPH_HUMAN | TdRPase | Thymidine Phosphorylase (TP) | Thymidine phosphorylase |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 49948.87 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | The recombinant human thymidine phosphorylase (V79TP) was expressed in V79 Chinese hamster cells (Sigma, T-9319). |
|---|
| Residue: | 482 |
|---|
| Sequence: | MAALMTPGTGAPPAPGDFSGEGSQGLPDPSPEPKQLPELIRMKRDGGRLSEADIRGFVAA
VVNGSAQGAQIGAMLMAIRLRGMDLEETSVLTQALAQSGQQLEWPEAWRQQLVDKHSTGG
VGDKVSLVLAPALAACGCKVPMISGRGLGHTGGTLDKLESIPGFNVIQSPEQMQVLLDQA
GCCIVGQSEQLVPADGILYAARDVTATVDSLPLITASILSKKLVEGLSALVVDVKFGGAA
VFPNQEQARELAKTLVGVGASLGLRVAAALTAMDKPLGRCVGHALEVEEALLCMDGAGPP
DLRDLVTTLGGALLWLSGHAGTQAQGAARVAAALDDGSALGRFERMLAAQGVDPGLARAL
CSGSPAERRQLLPRAREQEELLAPADGTVELVRALPLALVLHELGAGRSRAGEPLRLGVG
AELLVDVGQRLRRGTPWLRVHRDGPALSGPQSRALQEALVLSDRAPFAAPSPFAELVLPP
QQ
|
|
|
|---|
| BDBM50122764 |
|---|
| n/a |
|---|
| Name | BDBM50122764 |
|---|
| Synonyms: | 2-Amino-1-(5-bromo-2,6-dioxo-1,2,3,6-tetrahydro-pyrimidin-4-ylmethyl)-3H-imidazol-1-ium; chloride | 5-bromo-6-[(2-aminoimidazol-1-yl)methyl]uracil hydrochloride | CHEMBL122679 | CHEMBL65986 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C8H9BrN5O2 |
|---|
| Mol. Mass. | 287.093 |
|---|
| SMILES | Nc1[nH+]ccn1Cc1[nH]c(=O)[nH]c(=O)c1Br |
|---|
| Structure | 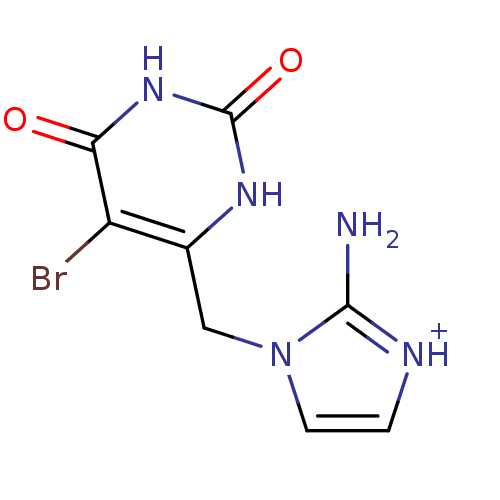
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Reigan, P; Edwards, PN; Gbaj, A; Cole, C; Barry, ST; Page, KM; Ashton, SE; Luke, RW; Douglas, KT; Stratford, IJ; Jaffar, M; Bryce, RA; Freeman, S Aminoimidazolylmethyluracil analogues as potent inhibitors of thymidine phosphorylase and their bioreductive nitroimidazolyl prodrugs. J Med Chem48:392-402 (2005) [PubMed] Article
Reigan, P; Edwards, PN; Gbaj, A; Cole, C; Barry, ST; Page, KM; Ashton, SE; Luke, RW; Douglas, KT; Stratford, IJ; Jaffar, M; Bryce, RA; Freeman, S Aminoimidazolylmethyluracil analogues as potent inhibitors of thymidine phosphorylase and their bioreductive nitroimidazolyl prodrugs. J Med Chem48:392-402 (2005) [PubMed] Article