| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Vasopressin V1a receptor |
|---|
| Ligand | BDBM50178214 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_326539 (CHEMBL863357) |
|---|
| Ki | 300±n/a nM |
|---|
| Citation |  Quattropani, A; Dorbais, J; Covini, D; Pittet, PA; Colovray, V; Thomas, RJ; Coxhead, R; Halazy, S; Scheer, A; Missotten, M; Ayala, G; Bradshaw, C; De Raemy-Schenk, AM; Nichols, A; Cirillo, R; Tos, EG; Giachetti, C; Golzio, L; Marinelli, P; Church, DJ; Barberis, C; Chollet, A; Schwarz, MK Discovery and development of a new class of potent, selective, orally active oxytocin receptor antagonists. J Med Chem48:7882-905 (2005) [PubMed] Article Quattropani, A; Dorbais, J; Covini, D; Pittet, PA; Colovray, V; Thomas, RJ; Coxhead, R; Halazy, S; Scheer, A; Missotten, M; Ayala, G; Bradshaw, C; De Raemy-Schenk, AM; Nichols, A; Cirillo, R; Tos, EG; Giachetti, C; Golzio, L; Marinelli, P; Church, DJ; Barberis, C; Chollet, A; Schwarz, MK Discovery and development of a new class of potent, selective, orally active oxytocin receptor antagonists. J Med Chem48:7882-905 (2005) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Vasopressin V1a receptor |
|---|
| Name: | Vasopressin V1a receptor |
|---|
| Synonyms: | AVPR V1a | AVPR1 | AVPR1A | Antidiuretic hormone receptor 1a | V1AR_HUMAN | V1aR | VASOPRESSIN V1A | Vascular/hepatic-type arginine vasopressin receptor | Vasopressin V1 receptor | Vasopressin V1a receptor | Vasopressin receptor |
|---|
| Type: | Receptor |
|---|
| Mol. Mass.: | 46820.18 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P37288 |
|---|
| Residue: | 418 |
|---|
| Sequence: | MRLSAGPDAGPSGNSSPWWPLATGAGNTSREAEALGEGNGPPRDVRNEELAKLEIAVLAV
TFAVAVLGNSSVLLALHRTPRKTSRMHLFIRHLSLADLAVAFFQVLPQMCWDITYRFRGP
DWLCRVVKHLQVFGMFASAYMLVVMTADRYIAVCHPLKTLQQPARRSRLMIAAAWVLSFV
LSTPQYFVFSMIEVNNVTKARDCWATFIQPWGSRAYVTWMTGGIFVAPVVILGTCYGFIC
YNIWCNVRGKTASRQSKGAEQAGVAFQKGFLLAPCVSSVKSISRAKIRTVKMTFVIVTAY
IVCWAPFFIIQMWSVWDPMSVWTESENPTITITALLGSLNSCCNPWIYMFFSGHLLQDCV
QSFPCCQNMKEKFNKEDTDSMSRRQTFYSNNRSPTNSTGMWKDSPKSSKSIKFIPVST
|
|
|
|---|
| BDBM50178214 |
|---|
| n/a |
|---|
| Name | BDBM50178214 |
|---|
| Synonyms: | 2-[Benzenesulfonyl-(4-methoxy-phenyl)-amino]-N-(3-chloro-2-methyl-phenyl)-acetamide | CHEMBL114302 | N-(3-chloro-2-methylphenyl)-2-(N-(4-methoxyphenyl)phenylsulfonamido)acetamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H21ClN2O4S |
|---|
| Mol. Mass. | 444.931 |
|---|
| SMILES | COc1ccc(cc1)N(CC(=O)Nc1cccc(Cl)c1C)S(=O)(=O)c1ccccc1 |
|---|
| Structure | 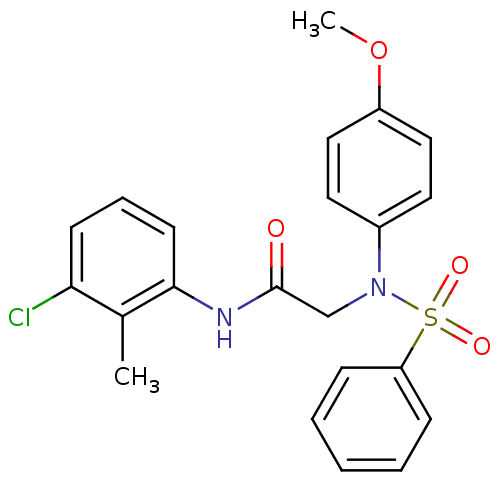
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Quattropani, A; Dorbais, J; Covini, D; Pittet, PA; Colovray, V; Thomas, RJ; Coxhead, R; Halazy, S; Scheer, A; Missotten, M; Ayala, G; Bradshaw, C; De Raemy-Schenk, AM; Nichols, A; Cirillo, R; Tos, EG; Giachetti, C; Golzio, L; Marinelli, P; Church, DJ; Barberis, C; Chollet, A; Schwarz, MK Discovery and development of a new class of potent, selective, orally active oxytocin receptor antagonists. J Med Chem48:7882-905 (2005) [PubMed] Article
Quattropani, A; Dorbais, J; Covini, D; Pittet, PA; Colovray, V; Thomas, RJ; Coxhead, R; Halazy, S; Scheer, A; Missotten, M; Ayala, G; Bradshaw, C; De Raemy-Schenk, AM; Nichols, A; Cirillo, R; Tos, EG; Giachetti, C; Golzio, L; Marinelli, P; Church, DJ; Barberis, C; Chollet, A; Schwarz, MK Discovery and development of a new class of potent, selective, orally active oxytocin receptor antagonists. J Med Chem48:7882-905 (2005) [PubMed] Article