| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Potassium voltage-gated channel subfamily H member 2 |
|---|
| Ligand | BDBM50181532 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_330885 (CHEMBL862040) |
|---|
| IC50 | >10000±n/a nM |
|---|
| Citation |  Jung, FH; Pasquet, G; Lambert-van der Brempt, C; Lohmann, JJ; Warin, N; Renaud, F; Germain, H; De Savi, C; Roberts, N; Johnson, T; Dousson, C; Hill, GB; Mortlock, AA; Heron, N; Wilkinson, RW; Wedge, SR; Heaton, SP; Odedra, R; Keen, NJ; Green, S; Brown, E; Thompson, K; Brightwell, S Discovery of novel and potent thiazoloquinazolines as selective Aurora A and B kinase inhibitors. J Med Chem49:955-70 (2006) [PubMed] Article Jung, FH; Pasquet, G; Lambert-van der Brempt, C; Lohmann, JJ; Warin, N; Renaud, F; Germain, H; De Savi, C; Roberts, N; Johnson, T; Dousson, C; Hill, GB; Mortlock, AA; Heron, N; Wilkinson, RW; Wedge, SR; Heaton, SP; Odedra, R; Keen, NJ; Green, S; Brown, E; Thompson, K; Brightwell, S Discovery of novel and potent thiazoloquinazolines as selective Aurora A and B kinase inhibitors. J Med Chem49:955-70 (2006) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Potassium voltage-gated channel subfamily H member 2 |
|---|
| Name: | Potassium voltage-gated channel subfamily H member 2 |
|---|
| Synonyms: | 1,3-beta-glucan synthase component GLS2 | Cytochrome P450 3A4 | ERG | ERG1 | Eag-related protein 1 | Ether a-go-go related gene potassium channel (hERG) | Ether-a-go-go-related gene (HERG) | Ether-a-go-go-related gene potassium channel (hERG) | Ether-a-go-go-related gene potassium channel 1 | Ether-a-go-go-related gene potassium channel 1 (HERG) | Ether-a-go-go-related gene potassium channel 1 (hERG1) | Ether-a-go-go-related protein (hERG) | Ether-a-go-go-related protein 1 | Ether-a-go-go-related protein 1 (HERG) | H-ERG | HERG | KCNH2 | KCNH2_HUMAN | Potassium voltage-gated channel subfamily H member 2 (hERG) | Transcriptional regulator ERG | Voltage-gated potassium channel subunit Kv11.1 | eag homolog | hERG Potassium Channel 1 | putative potassium channel subunit |
|---|
| Type: | Multi-pass membrane protein |
|---|
| Mol. Mass.: | 126672.65 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q12809 |
|---|
| Residue: | 1159 |
|---|
| Sequence: | MPVRRGHVAPQNTFLDTIIRKFEGQSRKFIIANARVENCAVIYCNDGFCELCGYSRAEVM
QRPCTCDFLHGPRTQRRAAAQIAQALLGAEERKVEIAFYRKDGSCFLCLVDVVPVKNEDG
AVIMFILNFEVVMEKDMVGSPAHDTNHRGPPTSWLAPGRAKTFRLKLPALLALTARESSV
RSGGAGGAGAPGAVVVDVDLTPAAPSSESLALDEVTAMDNHVAGLGPAEERRALVGPGSP
PRSAPGQLPSPRAHSLNPDASGSSCSLARTRSRESCASVRRASSADDIEAMRAGVLPPPP
RHASTGAMHPLRSGLLNSTSDSDLVRYRTISKIPQITLNFVDLKGDPFLASPTSDREIIA
PKIKERTHNVTEKVTQVLSLGADVLPEYKLQAPRIHRWTILHYSPFKAVWDWLILLLVIY
TAVFTPYSAAFLLKETEEGPPATECGYACQPLAVVDLIVDIMFIVDILINFRTTYVNANE
EVVSHPGRIAVHYFKGWFLIDMVAAIPFDLLIFGSGSEELIGLLKTARLLRLVRVARKLD
RYSEYGAAVLFLLMCTFALIAHWLACIWYAIGNMEQPHMDSRIGWLHNLGDQIGKPYNSS
GLGGPSIKDKYVTALYFTFSSLTSVGFGNVSPNTNSEKIFSICVMLIGSLMYASIFGNVS
AIIQRLYSGTARYHTQMLRVREFIRFHQIPNPLRQRLEEYFQHAWSYTNGIDMNAVLKGF
PECLQADICLHLNRSLLQHCKPFRGATKGCLRALAMKFKTTHAPPGDTLVHAGDLLTALY
FISRGSIEILRGDVVVAILGKNDIFGEPLNLYARPGKSNGDVRALTYCDLHKIHRDDLLE
VLDMYPEFSDHFWSSLEITFNLRDTNMIPGSPGSTELEGGFSRQRKRKLSFRRRTDKDTE
QPGEVSALGPGRAGAGPSSRGRPGGPWGESPSSGPSSPESSEDEGPGRSSSPLRLVPFSS
PRPPGEPPGGEPLMEDCEKSSDTCNPLSGAFSGVSNIFSFWGDSRGRQYQELPRCPAPTP
SLLNIPLSSPGRRPRGDVESRLDALQRQLNRLETRLSADMATVLQLLQRQMTLVPPAYSA
VTTPGPGPTSTSPLLPVSPLPTLTLDSLSQVSQFMACEELPPGAPELPQEGPTRRLSLPG
QLGALTSQPLHRHGSDPGS
|
|
|
|---|
| BDBM50181532 |
|---|
| n/a |
|---|
| Name | BDBM50181532 |
|---|
| Synonyms: | CHEMBL201307 | N-(3-fluorophenyl)-2-(2-(7-(3-(4-(hydroxymethyl)piperidin-1-yl)propoxy)-6-methoxyquinazolin-4-ylamino)thiazol-5-yl)acetamide | N-(3-fluorophenyl)-2-{2-[(7-{3-[4-(hydroxymethyl)piperidin-1-yl]propoxy}-6-methoxyquinazolin-4-yl)amino]-1,3-thiazol-5-yl}acetamide | N-3-fluorophenyl)-2-2-7-3-4-hydroxymethyl)piperidin-1-yl)propoxy)-6-methoxyquinazolin-4-ylamino)thiazol-5-yl)acetamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C29H33FN6O4S |
|---|
| Mol. Mass. | 580.674 |
|---|
| SMILES | COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(F)c4)s3)ncnc2cc1OCCCN1CCC(CO)CC1 |
|---|
| Structure | 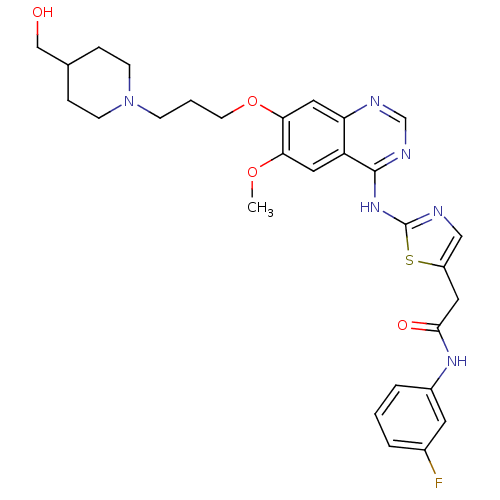
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Jung, FH; Pasquet, G; Lambert-van der Brempt, C; Lohmann, JJ; Warin, N; Renaud, F; Germain, H; De Savi, C; Roberts, N; Johnson, T; Dousson, C; Hill, GB; Mortlock, AA; Heron, N; Wilkinson, RW; Wedge, SR; Heaton, SP; Odedra, R; Keen, NJ; Green, S; Brown, E; Thompson, K; Brightwell, S Discovery of novel and potent thiazoloquinazolines as selective Aurora A and B kinase inhibitors. J Med Chem49:955-70 (2006) [PubMed] Article
Jung, FH; Pasquet, G; Lambert-van der Brempt, C; Lohmann, JJ; Warin, N; Renaud, F; Germain, H; De Savi, C; Roberts, N; Johnson, T; Dousson, C; Hill, GB; Mortlock, AA; Heron, N; Wilkinson, RW; Wedge, SR; Heaton, SP; Odedra, R; Keen, NJ; Green, S; Brown, E; Thompson, K; Brightwell, S Discovery of novel and potent thiazoloquinazolines as selective Aurora A and B kinase inhibitors. J Med Chem49:955-70 (2006) [PubMed] Article