| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5-hydroxytryptamine receptor 2A |
|---|
| Ligand | BDBM50073805 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_367041 (CHEMBL866804) |
|---|
| Ki | 380±n/a nM |
|---|
| Citation |  Enguehard-Gueiffier, C; Hübner, H; El Hakmaoui, A; Allouchi, H; Gmeiner, P; Argiolas, A; Melis, MR; Gueiffier, A 2-[(4-phenylpiperazin-1-yl)methyl]imidazo(di)azines as selective D4-ligands. Induction of penile erection by 2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl]imidazo[1,2-a]pyridine (PIP3EA), a potent and selective D4 partial agonist. J Med Chem49:3938-47 (2006) [PubMed] Article Enguehard-Gueiffier, C; Hübner, H; El Hakmaoui, A; Allouchi, H; Gmeiner, P; Argiolas, A; Melis, MR; Gueiffier, A 2-[(4-phenylpiperazin-1-yl)methyl]imidazo(di)azines as selective D4-ligands. Induction of penile erection by 2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl]imidazo[1,2-a]pyridine (PIP3EA), a potent and selective D4 partial agonist. J Med Chem49:3938-47 (2006) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5-hydroxytryptamine receptor 2A |
|---|
| Name: | 5-hydroxytryptamine receptor 2A |
|---|
| Synonyms: | 5-HT-2 | 5-HT-2A | 5-HT2 | 5-HT2A | 5HT2A_PIG | HTR2A | Serotonin 2a (5-HT2a) receptor |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 52679.13 |
|---|
| Organism: | PIG |
|---|
| Description: | 5-HT2 0 0::P50129 |
|---|
| Residue: | 470 |
|---|
| Sequence: | MDVLCEENTSLSSPTNSFMQLNDDTRLYHNDFNSGEANTSDAFNWTVDSENRTNLSCEGC
LSPPCFSLLHLQEKNWSALLTAVVIILTIAGNILVIMAVSLEKKLQNATNYFLMSLAIAD
MLLGFLVMPVSMLTILYGYRWPLPSKLCAVWIYLDVLFSTASIMHLCAISLDRYVAIQNP
IHHRRFNSRTKAFLKIIAVWTISVGISMPIPVFGLQDDSKVFKEGSCLLADDNFVLIGSF
VSFFIPLTIMVITYFLTIKSLQKEATLCVSDLGTRAKLASFSFLPQSSLSSEKLFQRSIH
REPGSYGRRTMQSISNEQKACKVLGIVFFLFVVMWCPFFITNIMAVICKESCNEDVIGAL
LNVFVWIGYLSSAVNPLVYTLFNKTYRSAFSRYIQCQYKENKKPLQLILVNTIPALAYKS
SQLQTGQKENSKQDDKATENDCTMVALGKQHSEDAPADNSNTVNEKVSCV
|
|
|
|---|
| BDBM50073805 |
|---|
| n/a |
|---|
| Name | BDBM50073805 |
|---|
| Synonyms: | 3-((4-(4-chlorophenyl)piperazin-1-yl)methyl)H-pyrazolo[1,5-a]pyridine | 3-[4-(4-Chloro-phenyl)-piperazin-1-ylmethyl]-pyrazolo[1,5-a]pyridine | 4-(4-Chloro-phenyl)-1-pyrazolo[1,5-a]pyridin-3-ylmethyl-piperazin-1-ium | CHEMBL7927 | FAUC 113 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H19ClN4 |
|---|
| Mol. Mass. | 326.823 |
|---|
| SMILES | Clc1ccc(cc1)N1CCN(Cc2cnn3ccccc23)CC1 |
|---|
| Structure | 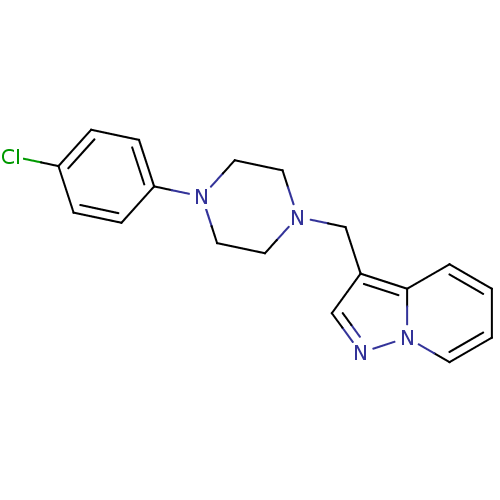
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Enguehard-Gueiffier, C; Hübner, H; El Hakmaoui, A; Allouchi, H; Gmeiner, P; Argiolas, A; Melis, MR; Gueiffier, A 2-[(4-phenylpiperazin-1-yl)methyl]imidazo(di)azines as selective D4-ligands. Induction of penile erection by 2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl]imidazo[1,2-a]pyridine (PIP3EA), a potent and selective D4 partial agonist. J Med Chem49:3938-47 (2006) [PubMed] Article
Enguehard-Gueiffier, C; Hübner, H; El Hakmaoui, A; Allouchi, H; Gmeiner, P; Argiolas, A; Melis, MR; Gueiffier, A 2-[(4-phenylpiperazin-1-yl)methyl]imidazo(di)azines as selective D4-ligands. Induction of penile erection by 2-[4-(2-methoxyphenyl)piperazin-1-ylmethyl]imidazo[1,2-a]pyridine (PIP3EA), a potent and selective D4 partial agonist. J Med Chem49:3938-47 (2006) [PubMed] Article