| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Tripeptidyl-peptidase 2 |
|---|
| Ligand | BDBM50176928 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_430943 (CHEMBL914885) |
|---|
| Ki | 900±n/a nM |
|---|
| Citation |  Ganellin, CR; Bishop, PB; Bambal, RB; Chan, SM; Leblond, B; Moore, AN; Zhao, L; Bourgeat, P; Rose, C; Vargas, F; Schwartz, JC Inhibitors of tripeptidyl peptidase II. 3. Derivation of butabindide by successive structure optimizations leading to a potential general approach to designing exopeptidase inhibitors. J Med Chem48:7333-42 (2005) [PubMed] Article Ganellin, CR; Bishop, PB; Bambal, RB; Chan, SM; Leblond, B; Moore, AN; Zhao, L; Bourgeat, P; Rose, C; Vargas, F; Schwartz, JC Inhibitors of tripeptidyl peptidase II. 3. Derivation of butabindide by successive structure optimizations leading to a potential general approach to designing exopeptidase inhibitors. J Med Chem48:7333-42 (2005) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Tripeptidyl-peptidase 2 |
|---|
| Name: | Tripeptidyl-peptidase 2 |
|---|
| Synonyms: | TPP2_RAT | Tpp2 | Tripeptidyl aminopeptidase |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 138287.08 |
|---|
| Organism: | Rattus norvegicus |
|---|
| Description: | ChEMBL_211948 |
|---|
| Residue: | 1249 |
|---|
| Sequence: | MATAATEEPFPFHGLLPKKETGASSFLCRYPEYDGRGVLIAVLDTGVDPGAPGMQVTTDG
KPKIIDIIDTTGSGDVNTATEVEPKDGEITGLSGRVLKIPANWTNPSGKYHIGIKNGYDF
YPKALKERIQKERKEKIWDPIHRVALAEACRKQEEFDIANNGSSQANKLIKEELQSQVEL
LNSFEKKYSDPGPVYDCLVWHDGETWRACVDSNENGDLGKSTVLRNYKEAQEYGSFGTAE
MLNYSVNIYDDGNLLSIVTSGGAHGTHVASIAAGHFPEEPERNGVAPGAQILSIKIGDTR
LSTMETGTGLIRAMIEVINHKCDLVNYSYGEATHWPNSGRICEVINEAVWKHNTIYVSSA
GNNGPCLSTVGCPGGTTSSVIGVGAYVSPDMMVAEYSLREKLPANQYTWSSRGPSADGAL
GVSISAPGGAIASVPNWTLRGTQLMNGTSMSSPNACGGIALVLSGLKANNVDYTVHSVRR
ALENTAIKADNIEVFAQGHGIIQVDKAYDYLIQNTSFANRLGFTVTVGNNRGIYLRDPVQ
VAAPSDHGVGIEPVFPENTENSEKISFQLHLALTSNSSWVQCPSHLELMNQCRHINIRVD
PRGLREGLHYTEVCGYDIASPNAGPLFRVPITAVIAAKVNESSHYDLAFTDVHFKPGQIR
RHFVEVPEGATWAEVTVCSCSSEVSAKFVLHAVQLVKQRAYRSHEFYKFCSLPEKGTLIE
AFPVLGGKAIEFCIARWWASLSDVNIDYTISFHGIVCTAPQLNIHASEGINRFDVQSSLK
YEDLAPCITLKSWVQTLRPVNAKTRPLGSRDVLPNNRQLYEMVLTYSFHQPKSGEVTPSC
PLLCELLYESEFDSQLWIIFDQNKRQMGSGDAYPHQYSLKLEKGDYTIRLQIRHEQISDL
DRLKDLPFIVSHRLSNTLSLDIHENHSLALLGKKKSSSLTLPPKYNQPFFVTSLPDDKIP
KGAGPGCYLAGSLTLSKTELGKKADVIPVHYYLIPPPTKTKNGSKDKEKDSEKEKDLKEE
FTEALRDLKIQWMTKLDSTDIYNELKETYPAYLPLYVARLHQLDAEKERMKRLNEIVDAA
NAVISHIDQTALAVYIAMKTDPRPDAATIKNDMDKQKSTLVDALCRKGCALADHLLHAQP
HDGAAAGDAEAKEEEGESTLESLSETYWETTKWTDLFDTKVLTFAYKHALVNKMYGRGLK
FATKLVEEKPTKENWKNCIQLMKLLGWTHCASFTENWLPIMYPPDYCVF
|
|
|
|---|
| BDBM50176928 |
|---|
| n/a |
|---|
| Name | BDBM50176928 |
|---|
| Synonyms: | CHEMBL375490 | L-valyl-L-proline hexylamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C16H31N3O2 |
|---|
| Mol. Mass. | 297.4362 |
|---|
| SMILES | CCCCCCNC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)C(C)C |r| |
|---|
| Structure | 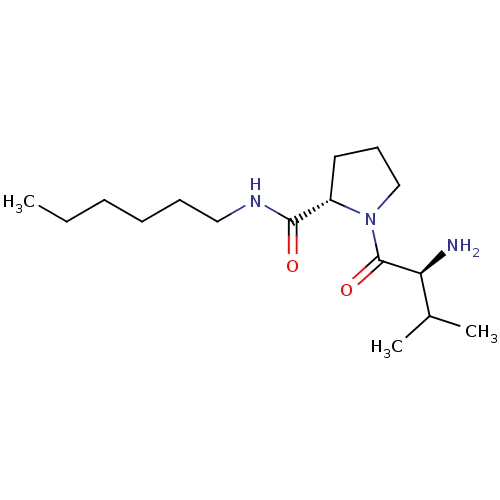
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ganellin, CR; Bishop, PB; Bambal, RB; Chan, SM; Leblond, B; Moore, AN; Zhao, L; Bourgeat, P; Rose, C; Vargas, F; Schwartz, JC Inhibitors of tripeptidyl peptidase II. 3. Derivation of butabindide by successive structure optimizations leading to a potential general approach to designing exopeptidase inhibitors. J Med Chem48:7333-42 (2005) [PubMed] Article
Ganellin, CR; Bishop, PB; Bambal, RB; Chan, SM; Leblond, B; Moore, AN; Zhao, L; Bourgeat, P; Rose, C; Vargas, F; Schwartz, JC Inhibitors of tripeptidyl peptidase II. 3. Derivation of butabindide by successive structure optimizations leading to a potential general approach to designing exopeptidase inhibitors. J Med Chem48:7333-42 (2005) [PubMed] Article