| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Tissue-type plasminogen activator |
|---|
| Ligand | BDBM50216556 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_447832 (CHEMBL898082) |
|---|
| Ki | 5502±n/a nM |
|---|
| Citation |  Qiao, JX; Chang, CH; Cheney, DL; Morin, PE; Wang, GZ; King, SR; Wang, TC; Rendina, AR; Luettgen, JM; Knabb, RM; Wexler, RR; Lam, PY SAR and X-ray structures of enantiopure 1,2-cis-(1R,2S)-cyclopentyldiamine and cyclohexyldiamine derivatives as inhibitors of coagulation Factor Xa. Bioorg Med Chem Lett17:4419-27 (2007) [PubMed] Article Qiao, JX; Chang, CH; Cheney, DL; Morin, PE; Wang, GZ; King, SR; Wang, TC; Rendina, AR; Luettgen, JM; Knabb, RM; Wexler, RR; Lam, PY SAR and X-ray structures of enantiopure 1,2-cis-(1R,2S)-cyclopentyldiamine and cyclohexyldiamine derivatives as inhibitors of coagulation Factor Xa. Bioorg Med Chem Lett17:4419-27 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Tissue-type plasminogen activator |
|---|
| Name: | Tissue-type plasminogen activator |
|---|
| Synonyms: | Alteplase | PLAT | Reteplase | TPA_HUMAN | Thrombin receptor protein | Tissue-type plasminogen activator | Tissue-type plasminogen activator (tPA) | Tissue-type plasminogen activator precursor | t-PA | t-Plasminogen Activator (tPA) | t-plasminogen activator |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 62931.08 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 562 |
|---|
| Sequence: | MDAMKRGLCCVLLLCGAVFVSPSQEIHARFRRGARSYQVICRDEKTQMIYQQHQSWLRPV
LRSNRVEYCWCNSGRAQCHSVPVKSCSEPRCFNGGTCQQALYFSDFVCQCPEGFAGKCCE
IDTRATCYEDQGISYRGTWSTAESGAECTNWNSSALAQKPYSGRRPDAIRLGLGNHNYCR
NPDRDSKPWCYVFKAGKYSSEFCSTPACSEGNSDCYFGNGSAYRGTHSLTESGASCLPWN
SMILIGKVYTAQNPSAQALGLGKHNYCRNPDGDAKPWCHVLKNRRLTWEYCDVPSCSTCG
LRQYSQPQFRIKGGLFADIASHPWQAAIFAKHRRSPGERFLCGGILISSCWILSAAHCFQ
ERFPPHHLTVILGRTYRVVPGEEEQKFEVEKYIVHKEFDDDTYDNDIALLQLKSDSSRCA
QESSVVRTVCLPPADLQLPDWTECELSGYGKHEALSPFYSERLKEAHVRLYPSSRCTSQH
LLNRTVTDNMLCAGDTRSGGPQANLHDACQGDSGGPLVCLNDGRMTLVGIISWGLGCGQK
DVPGVYTKVTNYLDWIRDNMRP
|
|
|
|---|
| BDBM50216556 |
|---|
| n/a |
|---|
| Name | BDBM50216556 |
|---|
| Synonyms: | 3-CHLORO-N-((1R,2S) -2-(4-(2-OXOPYRIDIN-1(2H)-YL)BENZAMIDO)CYCLOHEXYL)-1H-INDOLE-6-CARBOXAMIDE | 3-chloro-N-((1R,2S)-2-(4-(2-oxopyridin-1(2H)-yl)benzamido)cyclohexyl)-1H-indole-6-carboxamide | CHEMBL398380 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C27H25ClN4O3 |
|---|
| Mol. Mass. | 488.965 |
|---|
| SMILES | Clc1c[nH]c2cc(ccc12)C(=O)N[C@@H]1CCCC[C@@H]1NC(=O)c1ccc(cc1)-n1ccccc1=O |
|---|
| Structure | 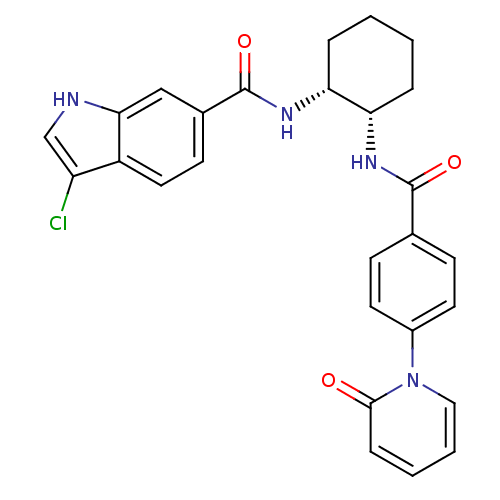
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Qiao, JX; Chang, CH; Cheney, DL; Morin, PE; Wang, GZ; King, SR; Wang, TC; Rendina, AR; Luettgen, JM; Knabb, RM; Wexler, RR; Lam, PY SAR and X-ray structures of enantiopure 1,2-cis-(1R,2S)-cyclopentyldiamine and cyclohexyldiamine derivatives as inhibitors of coagulation Factor Xa. Bioorg Med Chem Lett17:4419-27 (2007) [PubMed] Article
Qiao, JX; Chang, CH; Cheney, DL; Morin, PE; Wang, GZ; King, SR; Wang, TC; Rendina, AR; Luettgen, JM; Knabb, RM; Wexler, RR; Lam, PY SAR and X-ray structures of enantiopure 1,2-cis-(1R,2S)-cyclopentyldiamine and cyclohexyldiamine derivatives as inhibitors of coagulation Factor Xa. Bioorg Med Chem Lett17:4419-27 (2007) [PubMed] Article