| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Acetylcholinesterase |
|---|
| Ligand | BDBM50211241 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_455571 (CHEMBL886351) |
|---|
| IC50 | 2.47±n/a nM |
|---|
| Citation |  Tang, H; Ning, FX; Wei, YB; Huang, SL; Huang, ZS; Chan, AS; Gu, LQ Derivatives of oxoisoaporphine alkaloids: a novel class of selective acetylcholinesterase inhibitors. Bioorg Med Chem Lett17:3765-8 (2007) [PubMed] Article Tang, H; Ning, FX; Wei, YB; Huang, SL; Huang, ZS; Chan, AS; Gu, LQ Derivatives of oxoisoaporphine alkaloids: a novel class of selective acetylcholinesterase inhibitors. Bioorg Med Chem Lett17:3765-8 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Acetylcholinesterase |
|---|
| Name: | Acetylcholinesterase |
|---|
| Synonyms: | ACES_ELEEL | Acetylcholinesterase (AChE) | Acetylcholinesterase (EeAChE) | ache |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 71812.79 |
|---|
| Organism: | Electrophorus electricus (Electric eel) |
|---|
| Description: | n/a |
|---|
| Residue: | 633 |
|---|
| Sequence: | MKILDALLFPVIFIMFFIHLSIAQTDPELTIMTRLGQVQGTRLPVPDRSHVIAFLGIPFA
EPPLGKMRFKPPEPKKPWNDVFDARDYPSACYQYVDTSYPGFSGTEMWNPNRMMSEDCLY
LNVWVPATPRPHNLTVMVWIYGGGFYSGSSSLDVYDGRYLAHSEKVVVVSMNYRVSAFGF
LALNGSAEAPGNVGLLDQRLALQWVQDNIHFFGGNPKQVTIFGESAGAASVGMHLLSPDS
RPKFTRAILQSGVPNGPWRTVSFDEARRRAIKLGRLVGCPDGNDTDLIDCLRSKQPQDLI
DQEWLVLPFSGLFRFSFVPVIDGVVFPDTPEAMLNSGNFKDTQILLGVNQNEGSYFLIYG
APGFSKDNESLITREDFLQGVKMSVPHANEIGLEAVILQYTDWMDEDNPIKNREAMDDIV
GDHNVVCPLQHFAKMYAQYSILQGQTGTASQGNLGWGNSGSASNSGNSQVSVYLYMFDHR
ASNLVWPEWMGVIHGYEIEFVFGLPLEKRLNYTLEEEKLSRRMMKYWANFARTGNPNINV
DGSIDSRRRWPVFTSTEQKHVGLNTDSLKVHKGLKSQFCALWNRFLPRLLNVTENIDDAE
RQWKAEFHRWSSYMMHWKNQFDHYSKQERCTNL
|
|
|
|---|
| BDBM50211241 |
|---|
| n/a |
|---|
| Name | BDBM50211241 |
|---|
| Synonyms: | 9-[3-Pyrrolidinopropionamido]-1-azabenzanthrone | CHEMBL246911 | N-(7-oxo-7H-1-aza-benzo[de]anthracen-9-yl)-3-pyrrolidin-1-yl-propionamide |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H21N3O2 |
|---|
| Mol. Mass. | 371.4317 |
|---|
| SMILES | O=C(CCN1CCCC1)Nc1ccc-2c(c1)C(=O)c1cccc3ccnc-2c13 |
|---|
| Structure | 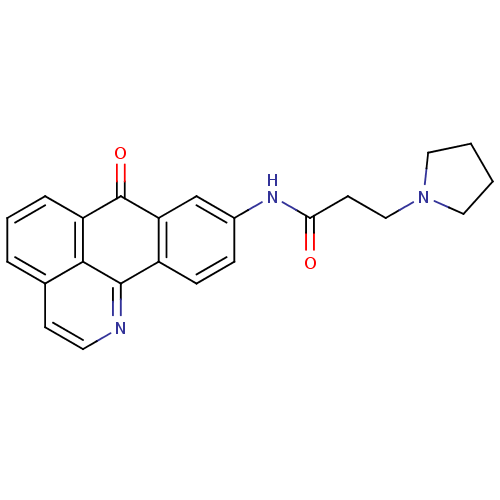
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Tang, H; Ning, FX; Wei, YB; Huang, SL; Huang, ZS; Chan, AS; Gu, LQ Derivatives of oxoisoaporphine alkaloids: a novel class of selective acetylcholinesterase inhibitors. Bioorg Med Chem Lett17:3765-8 (2007) [PubMed] Article
Tang, H; Ning, FX; Wei, YB; Huang, SL; Huang, ZS; Chan, AS; Gu, LQ Derivatives of oxoisoaporphine alkaloids: a novel class of selective acetylcholinesterase inhibitors. Bioorg Med Chem Lett17:3765-8 (2007) [PubMed] Article