| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Receptor-type tyrosine-protein kinase FLT3 |
|---|
| Ligand | BDBM50224883 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_458443 (CHEMBL941761) |
|---|
| Ki | >2481±n/a nM |
|---|
| Citation |  Tao, ZF; Chen, Z; Bui, MH; Kovar, P; Johnson, E; Bouska, J; Zhang, H; Rosenberg, S; Sowin, T; Lin, NH Macrocyclic ureas as potent and selective Chk1 inhibitors: an improved synthesis, kinome profiling, structure-activity relationships, and preliminary pharmacokinetics. Bioorg Med Chem Lett17:6593-601 (2007) [PubMed] Article Tao, ZF; Chen, Z; Bui, MH; Kovar, P; Johnson, E; Bouska, J; Zhang, H; Rosenberg, S; Sowin, T; Lin, NH Macrocyclic ureas as potent and selective Chk1 inhibitors: an improved synthesis, kinome profiling, structure-activity relationships, and preliminary pharmacokinetics. Bioorg Med Chem Lett17:6593-601 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Receptor-type tyrosine-protein kinase FLT3 |
|---|
| Name: | Receptor-type tyrosine-protein kinase FLT3 |
|---|
| Synonyms: | CD135 | CD_antigen: CD135 | FL cytokine receptor | FLK-2 | FLK2 | FLT-3 | FLT3 | FLT3_HUMAN | Fetal liver kinase-2 | Fms-like tyrosine kinase 3 | Fms-like tyrosine kinase 3 (Flt-3) | Fms-related tyrosine kinase 3 | STK-1 | STK1 | Stem cell tyrosine kinase 1 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 112888.62 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P36888 |
|---|
| Residue: | 993 |
|---|
| Sequence: | MPALARDGGQLPLLVVFSAMIFGTITNQDLPVIKCVLINHKNNDSSVGKSSSYPMVSESP
EDLGCALRPQSSGTVYEAAAVEVDVSASITLQVLVDAPGNISCLWVFKHSSLNCQPHFDL
QNRGVVSMVILKMTETQAGEYLLFIQSEATNYTILFTVSIRNTLLYTLRRPYFRKMENQD
ALVCISESVPEPIVEWVLCDSQGESCKEESPAVVKKEEKVLHELFGTDIRCCARNELGRE
CTRLFTIDLNQTPQTTLPQLFLKVGEPLWIRCKAVHVNHGFGLTWELENKALEEGNYFEM
STYSTNRTMIRILFAFVSSVARNDTGYYTCSSSKHPSQSALVTIVEKGFINATNSSEDYE
IDQYEEFCFSVRFKAYPQIRCTWTFSRKSFPCEQKGLDNGYSISKFCNHKHQPGEYIFHA
ENDDAQFTKMFTLNIRRKPQVLAEASASQASCFSDGYPLPSWTWKKCSDKSPNCTEEITE
GVWNRKANRKVFGQWVSSSTLNMSEAIKGFLVKCCAYNSLGTSCETILLNSPGPFPFIQD
NISFYATIGVCLLFIVVLTLLICHKYKKQFRYESQLQMVQVTGSSDNEYFYVDFREYEYD
LKWEFPRENLEFGKVLGSGAFGKVMNATAYGISKTGVSIQVAVKMLKEKADSSEREALMS
ELKMMTQLGSHENIVNLLGACTLSGPIYLIFEYCCYGDLLNYLRSKREKFHRTWTEIFKE
HNFSFYPTFQSHPNSSMPGSREVQIHPDSDQISGLHGNSFHSEDEIEYENQKRLEEEEDL
NVLTFEDLLCFAYQVAKGMEFLEFKSCVHRDLAARNVLVTHGKVVKICDFGLARDIMSDS
NYVVRGNARLPVKWMAPESLFEGIYTIKSDVWSYGILLWEIFSLGVNPYPGIPVDANFYK
LIQNGFKMDQPFYATEEIYIIMQSCWAFDSRKRPSFPNLTSFLGCQLADAEEAMYQNVDG
RVSECPHTYQNRRPFSREMDLGLLSPQAQVEDS
|
|
|
|---|
| BDBM50224883 |
|---|
| n/a |
|---|
| Name | BDBM50224883 |
|---|
| Synonyms: | 7-chloro-3-oxo-8-[(thiazol-5-ylmethyl)-amino]-11,16-dioxa-2,4,19,21-tetraaza-tricyclo[15.3.1.0*5,10*]henicosa-1(21),5,7,9,17,19-hexaene-18-crbonitrile | CHEMBL248757 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H18ClN7O3S |
|---|
| Mol. Mass. | 471.92 |
|---|
| SMILES | Clc1cc2NC(=O)Nc3cnc(C#N)c(OCCCCOc2cc1NCc1cncs1)n3 |
|---|
| Structure | 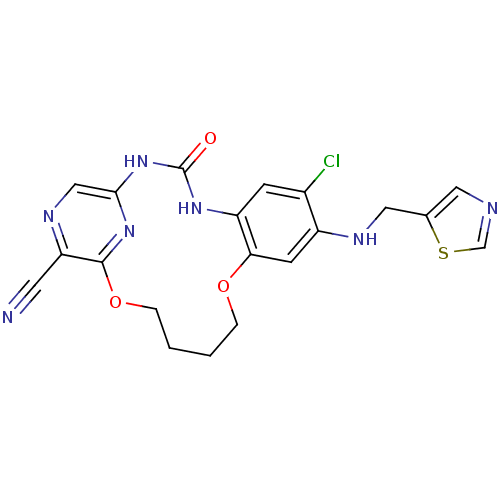
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Tao, ZF; Chen, Z; Bui, MH; Kovar, P; Johnson, E; Bouska, J; Zhang, H; Rosenberg, S; Sowin, T; Lin, NH Macrocyclic ureas as potent and selective Chk1 inhibitors: an improved synthesis, kinome profiling, structure-activity relationships, and preliminary pharmacokinetics. Bioorg Med Chem Lett17:6593-601 (2007) [PubMed] Article
Tao, ZF; Chen, Z; Bui, MH; Kovar, P; Johnson, E; Bouska, J; Zhang, H; Rosenberg, S; Sowin, T; Lin, NH Macrocyclic ureas as potent and selective Chk1 inhibitors: an improved synthesis, kinome profiling, structure-activity relationships, and preliminary pharmacokinetics. Bioorg Med Chem Lett17:6593-601 (2007) [PubMed] Article