| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Receptor tyrosine-protein kinase erbB-2 |
|---|
| Ligand | BDBM50224883 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_458437 (CHEMBL941755) |
|---|
| Ki | >1438±n/a nM |
|---|
| Citation |  Tao, ZF; Chen, Z; Bui, MH; Kovar, P; Johnson, E; Bouska, J; Zhang, H; Rosenberg, S; Sowin, T; Lin, NH Macrocyclic ureas as potent and selective Chk1 inhibitors: an improved synthesis, kinome profiling, structure-activity relationships, and preliminary pharmacokinetics. Bioorg Med Chem Lett17:6593-601 (2007) [PubMed] Article Tao, ZF; Chen, Z; Bui, MH; Kovar, P; Johnson, E; Bouska, J; Zhang, H; Rosenberg, S; Sowin, T; Lin, NH Macrocyclic ureas as potent and selective Chk1 inhibitors: an improved synthesis, kinome profiling, structure-activity relationships, and preliminary pharmacokinetics. Bioorg Med Chem Lett17:6593-601 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Receptor tyrosine-protein kinase erbB-2 |
|---|
| Name: | Receptor tyrosine-protein kinase erbB-2 |
|---|
| Synonyms: | 2.7.10.1 | C-erbB-2 | CD_antigen=CD340 | ERBB2 | ERBB2_HUMAN | ErbB-2/ErbB-3 heterodimer | FASN/HER2 | HER-2 Substrate | HER2 | MLN 19 | MLN19 | Metastatic lymph node gene 19 protein | NEU | NGL | Proto-oncogene Neu | Proto-oncogene c-ErbB-2 | Tyrosine kinase-type cell surface receptor HER2 | p185erbB2 |
|---|
| Type: | n/a |
|---|
| Mol. Mass.: | 137894.47 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P04626 |
|---|
| Residue: | 1255 |
|---|
| Sequence: | MELAALCRWGLLLALLPPGAASTQVCTGTDMKLRLPASPETHLDMLRHLYQGCQVVQGNL
ELTYLPTNASLSFLQDIQEVQGYVLIAHNQVRQVPLQRLRIVRGTQLFEDNYALAVLDNG
DPLNNTTPVTGASPGGLRELQLRSLTEILKGGVLIQRNPQLCYQDTILWKDIFHKNNQLA
LTLIDTNRSRACHPCSPMCKGSRCWGESSEDCQSLTRTVCAGGCARCKGPLPTDCCHEQC
AAGCTGPKHSDCLACLHFNHSGICELHCPALVTYNTDTFESMPNPEGRYTFGASCVTACP
YNYLSTDVGSCTLVCPLHNQEVTAEDGTQRCEKCSKPCARVCYGLGMEHLREVRAVTSAN
IQEFAGCKKIFGSLAFLPESFDGDPASNTAPLQPEQLQVFETLEEITGYLYISAWPDSLP
DLSVFQNLQVIRGRILHNGAYSLTLQGLGISWLGLRSLRELGSGLALIHHNTHLCFVHTV
PWDQLFRNPHQALLHTANRPEDECVGEGLACHQLCARGHCWGPGPTQCVNCSQFLRGQEC
VEECRVLQGLPREYVNARHCLPCHPECQPQNGSVTCFGPEADQCVACAHYKDPPFCVARC
PSGVKPDLSYMPIWKFPDEEGACQPCPINCTHSCVDLDDKGCPAEQRASPLTSIISAVVG
ILLVVVLGVVFGILIKRRQQKIRKYTMRRLLQETELVEPLTPSGAMPNQAQMRILKETEL
RKVKVLGSGAFGTVYKGIWIPDGENVKIPVAIKVLRENTSPKANKEILDEAYVMAGVGSP
YVSRLLGICLTSTVQLVTQLMPYGCLLDHVRENRGRLGSQDLLNWCMQIAKGMSYLEDVR
LVHRDLAARNVLVKSPNHVKITDFGLARLLDIDETEYHADGGKVPIKWMALESILRRRFT
HQSDVWSYGVTVWELMTFGAKPYDGIPAREIPDLLEKGERLPQPPICTIDVYMIMVKCWM
IDSECRPRFRELVSEFSRMARDPQRFVVIQNEDLGPASPLDSTFYRSLLEDDDMGDLVDA
EEYLVPQQGFFCPDPAPGAGGMVHHRHRSSSTRSGGGDLTLGLEPSEEEAPRSPLAPSEG
AGSDVFDGDLGMGAAKGLQSLPTHDPSPLQRYSEDPTVPLPSETDGYVAPLTCSPQPEYV
NQPDVRPQPPSPREGPLPAARPAGATLERPKTLSPGKNGVVKDVFAFGGAVENPEYLTPQ
GGAAPQPHPPPAFSPAFDNLYYWDQDPPERGAPPSTFKGTPTAENPEYLGLDVPV
|
|
|
|---|
| BDBM50224883 |
|---|
| n/a |
|---|
| Name | BDBM50224883 |
|---|
| Synonyms: | 7-chloro-3-oxo-8-[(thiazol-5-ylmethyl)-amino]-11,16-dioxa-2,4,19,21-tetraaza-tricyclo[15.3.1.0*5,10*]henicosa-1(21),5,7,9,17,19-hexaene-18-crbonitrile | CHEMBL248757 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H18ClN7O3S |
|---|
| Mol. Mass. | 471.92 |
|---|
| SMILES | Clc1cc2NC(=O)Nc3cnc(C#N)c(OCCCCOc2cc1NCc1cncs1)n3 |
|---|
| Structure | 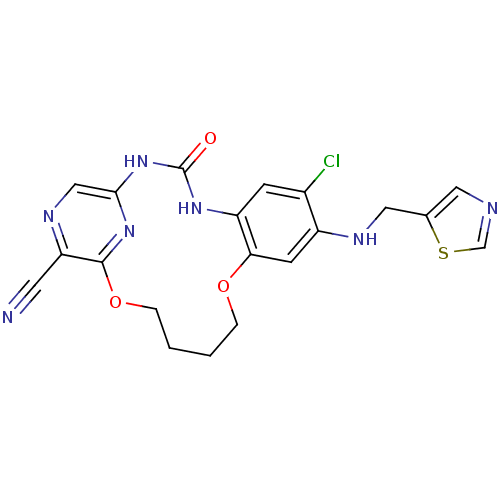
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Tao, ZF; Chen, Z; Bui, MH; Kovar, P; Johnson, E; Bouska, J; Zhang, H; Rosenberg, S; Sowin, T; Lin, NH Macrocyclic ureas as potent and selective Chk1 inhibitors: an improved synthesis, kinome profiling, structure-activity relationships, and preliminary pharmacokinetics. Bioorg Med Chem Lett17:6593-601 (2007) [PubMed] Article
Tao, ZF; Chen, Z; Bui, MH; Kovar, P; Johnson, E; Bouska, J; Zhang, H; Rosenberg, S; Sowin, T; Lin, NH Macrocyclic ureas as potent and selective Chk1 inhibitors: an improved synthesis, kinome profiling, structure-activity relationships, and preliminary pharmacokinetics. Bioorg Med Chem Lett17:6593-601 (2007) [PubMed] Article