| Reaction Details |
|---|
 | Report a problem with these data |
| Target | 5'-nucleotidase |
|---|
| Ligand | BDBM50336767 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_463991 (CHEMBL929978) |
|---|
| Ki | >100000±n/a nM |
|---|
| Citation |  Weyler, S; Baqi, Y; Hillmann, P; Kaulich, M; Hunder, AM; Müller, IA; Müller, CE Combinatorial synthesis of anilinoanthraquinone derivatives and evaluation as non-nucleotide-derived P2Y2 receptor antagonists. Bioorg Med Chem Lett18:223-7 (2008) [PubMed] Article Weyler, S; Baqi, Y; Hillmann, P; Kaulich, M; Hunder, AM; Müller, IA; Müller, CE Combinatorial synthesis of anilinoanthraquinone derivatives and evaluation as non-nucleotide-derived P2Y2 receptor antagonists. Bioorg Med Chem Lett18:223-7 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| 5'-nucleotidase |
|---|
| Name: | 5'-nucleotidase |
|---|
| Synonyms: | 5'-nucleotidase | 5NTD_RAT | Ecto-5'-nucleotidase (e5'NT) | Ecto-5-nucleotidase (e5'NT) | NT | Nt5 | Nt5e | Nte |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 63971.44 |
|---|
| Organism: | Rattus norvegicus (Rat) |
|---|
| Description: | P21588 |
|---|
| Residue: | 576 |
|---|
| Sequence: | MRPAAATAPKWLLLALSALLPLWPTAKSWELTIMHTNDVHSRLEQTSDDSTKCLNASLCV
GGVARLFTKVQQIRKEEPNVLLLDAGDQYQGTIWFTVYKGLEVAHFMNLLGYDAMALGNH
EFDNGVEGLIDPLLRNVKFPILSANIKARGPLAPQISGLYLPYKVLSVGGEVVGIVGYTS
KETPFLSNPGTNLVFEDEVTALQPEVDKLKTLNVNKIIALGHSGFEMDKLIAQKVRGVDV
VVGGHTNTFLYTGNPPSKEVPAGKYPFIVTSDDGRKVPVVQAYAFGKYLGYLKVEFDDKG
NVVTSYGNPILLNSTIREDAAIKADINQWRIKLDNYSTQELGRTIVYLNGSAQECRFREC
NMGNLICDAMINNNLRHPDEMFWNHVSMCIVNGGGIRSPIDERNNGTITWENLAAVLPFG
GTFDLVQLKGSTLKKAFEHSVHRYGQSTGEFLQVGGIHVVYDISRKPWDRVVQLKVLCTK
CRVPIYEPLEMDKVYKVVLPSYLVNGGDGFQMIKDELLKHDSGDQDISVVSEYISKMKVI
YPAVEGRIKFSAASHYQGSFPLIILSFWAVILVLYQ
|
|
|
|---|
| BDBM50336767 |
|---|
| n/a |
|---|
| Name | BDBM50336767 |
|---|
| Synonyms: | CHEMBL257495 | PSB-716 | sodium 1-amino-4-(2-methoxyphenylamino)-9,10-dioxo-9,10-dihydroanthracene-2-sulfonate |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H15N2O6S |
|---|
| Mol. Mass. | 423.419 |
|---|
| SMILES | COc1ccccc1Nc1cc(c(N)c2C(=O)c3ccccc3C(=O)c12)S([O-])(=O)=O |
|---|
| Structure | 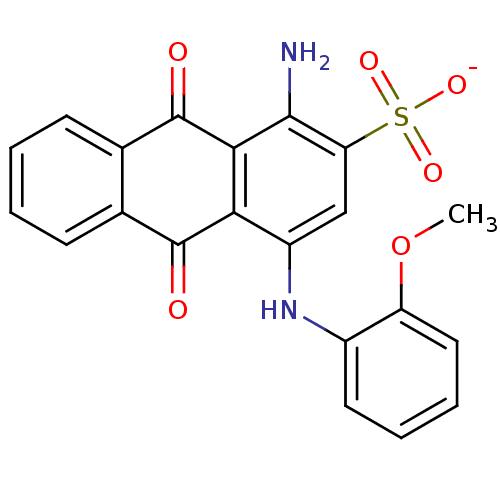
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Weyler, S; Baqi, Y; Hillmann, P; Kaulich, M; Hunder, AM; Müller, IA; Müller, CE Combinatorial synthesis of anilinoanthraquinone derivatives and evaluation as non-nucleotide-derived P2Y2 receptor antagonists. Bioorg Med Chem Lett18:223-7 (2008) [PubMed] Article
Weyler, S; Baqi, Y; Hillmann, P; Kaulich, M; Hunder, AM; Müller, IA; Müller, CE Combinatorial synthesis of anilinoanthraquinone derivatives and evaluation as non-nucleotide-derived P2Y2 receptor antagonists. Bioorg Med Chem Lett18:223-7 (2008) [PubMed] Article