| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Prostaglandin G/H synthase 2 |
|---|
| Ligand | BDBM50376865 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_478523 (CHEMBL923485) |
|---|
| IC50 | 850±n/a nM |
|---|
| Citation |  Reddy, MV; Billa, VK; Pallela, VR; Mallireddigari, MR; Boominathan, R; Gabriel, JL; Reddy, EP Design, synthesis, and biological evaluation of 1-(4-sulfamylphenyl)-3-trifluoromethyl-5-indolyl pyrazolines as cyclooxygenase-2 (COX-2) and lipoxygenase (LOX) inhibitors. Bioorg Med Chem16:3907-16 (2008) [PubMed] Article Reddy, MV; Billa, VK; Pallela, VR; Mallireddigari, MR; Boominathan, R; Gabriel, JL; Reddy, EP Design, synthesis, and biological evaluation of 1-(4-sulfamylphenyl)-3-trifluoromethyl-5-indolyl pyrazolines as cyclooxygenase-2 (COX-2) and lipoxygenase (LOX) inhibitors. Bioorg Med Chem16:3907-16 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Prostaglandin G/H synthase 2 |
|---|
| Name: | Prostaglandin G/H synthase 2 |
|---|
| Synonyms: | COX2 | Cyclooxygenase-1 (COX-1) | Cyclooxygenase-2 (COX-2) | PGH2_SHEEP | PTGS2 | Prostaglandin G/H synthase (Cyclooxygenase-2) | Prostaglandin G/H synthase (cyclooxygenase) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 68976.98 |
|---|
| Organism: | Ovis aries (Sheep) |
|---|
| Description: | n/a |
|---|
| Residue: | 603 |
|---|
| Sequence: | MLARALLLCAAVVCGAANPCCSHPCQNRGVCMSVGFDQYKCDCTRTGFYGENCTTPEFLT
RIKLLLKPTPDTVHYILTHFKGVWNIVNKISFLRNMIMRYVLTSRSHLIESPPTYNVHYS
YKSWEAFSNLSYYTRALPPVPDDCPTPMGVKGRKELPDSKEVVKKVLLRRKFIPDPQGTN
LMFAFFAQHFTHQFFKTDIERGPAFTKGKNHGVDLSHVYGESLERQHNRRLFKDGKMKYQ
MINGEMYPPTVKDTQVEMIYPPHIPEHLKFAVGQEVFGLVPGLMMYATIWLREHNRVCDV
LKQEHPEWGDEQLFQTSRLILIGETIKIVIEDYVQHLSGYHFKLKFDPELLFNQQFQYQN
RIAAEFNTLYHWHPLLPDVFQIDGQEYNYQQFIYNNSVLLEHGVTQFVESFTRQIAGRVA
GRRNLPAAVEKVSKASLDQSREMKYQSFNEYRKRFLLKPYESFEELTGEKEMAAELEALY
GDIDAMELYPALLVEKPAPDAIFGETMVEAGAPFSLKGLMGNPICSPEYWKPSTFGGEVG
FKIINTASIQSLICSNVKGCPFTSFSVQDAHLTKTVTINASSSHSGLDDINPTVLLKERS
TEL
|
|
|
|---|
| BDBM50376865 |
|---|
| n/a |
|---|
| Name | BDBM50376865 |
|---|
| Synonyms: | CHEMBL412100 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C18H16F3N5O2S |
|---|
| Mol. Mass. | 423.412 |
|---|
| SMILES | Nc1ccc2[nH]cc(C3CC(=NN3c3ccc(cc3)S(N)(=O)=O)C(F)(F)F)c2c1 |c:10| |
|---|
| Structure | 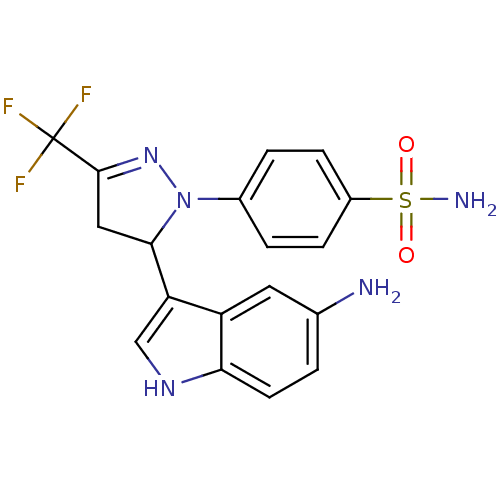
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Reddy, MV; Billa, VK; Pallela, VR; Mallireddigari, MR; Boominathan, R; Gabriel, JL; Reddy, EP Design, synthesis, and biological evaluation of 1-(4-sulfamylphenyl)-3-trifluoromethyl-5-indolyl pyrazolines as cyclooxygenase-2 (COX-2) and lipoxygenase (LOX) inhibitors. Bioorg Med Chem16:3907-16 (2008) [PubMed] Article
Reddy, MV; Billa, VK; Pallela, VR; Mallireddigari, MR; Boominathan, R; Gabriel, JL; Reddy, EP Design, synthesis, and biological evaluation of 1-(4-sulfamylphenyl)-3-trifluoromethyl-5-indolyl pyrazolines as cyclooxygenase-2 (COX-2) and lipoxygenase (LOX) inhibitors. Bioorg Med Chem16:3907-16 (2008) [PubMed] Article