| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Receptor tyrosine-protein kinase erbB-2 |
|---|
| Ligand | BDBM50161957 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_531104 (CHEMBL977765) |
|---|
| EC50 | 81±n/a nM |
|---|
| Citation |  McDermott, U; Sharma, SV; Dowell, L; Greninger, P; Montagut, C; Lamb, J; Archibald, H; Raudales, R; Tam, A; Lee, D; Rothenberg, SM; Supko, JG; Sordella, R; Ulkus, LE; Iafrate, AJ; Maheswaran, S; Njauw, CN; Tsao, H; Drew, L; Hanke, JH; Ma, XJ; Erlander, MG; Gray, NS; Haber, DA; Settleman, J Identification of genotype-correlated sensitivity to selective kinase inhibitors by using high-throughput tumor cell line profiling. Proc Natl Acad Sci U S A104:19936-41 (2007) [PubMed] Article McDermott, U; Sharma, SV; Dowell, L; Greninger, P; Montagut, C; Lamb, J; Archibald, H; Raudales, R; Tam, A; Lee, D; Rothenberg, SM; Supko, JG; Sordella, R; Ulkus, LE; Iafrate, AJ; Maheswaran, S; Njauw, CN; Tsao, H; Drew, L; Hanke, JH; Ma, XJ; Erlander, MG; Gray, NS; Haber, DA; Settleman, J Identification of genotype-correlated sensitivity to selective kinase inhibitors by using high-throughput tumor cell line profiling. Proc Natl Acad Sci U S A104:19936-41 (2007) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Receptor tyrosine-protein kinase erbB-2 |
|---|
| Name: | Receptor tyrosine-protein kinase erbB-2 |
|---|
| Synonyms: | 2.7.10.1 | C-erbB-2 | CD_antigen=CD340 | ERBB2 | ERBB2_HUMAN | ErbB-2/ErbB-3 heterodimer | FASN/HER2 | HER-2 Substrate | HER2 | MLN 19 | MLN19 | Metastatic lymph node gene 19 protein | NEU | NGL | Proto-oncogene Neu | Proto-oncogene c-ErbB-2 | Tyrosine kinase-type cell surface receptor HER2 | p185erbB2 |
|---|
| Type: | n/a |
|---|
| Mol. Mass.: | 137894.47 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P04626 |
|---|
| Residue: | 1255 |
|---|
| Sequence: | MELAALCRWGLLLALLPPGAASTQVCTGTDMKLRLPASPETHLDMLRHLYQGCQVVQGNL
ELTYLPTNASLSFLQDIQEVQGYVLIAHNQVRQVPLQRLRIVRGTQLFEDNYALAVLDNG
DPLNNTTPVTGASPGGLRELQLRSLTEILKGGVLIQRNPQLCYQDTILWKDIFHKNNQLA
LTLIDTNRSRACHPCSPMCKGSRCWGESSEDCQSLTRTVCAGGCARCKGPLPTDCCHEQC
AAGCTGPKHSDCLACLHFNHSGICELHCPALVTYNTDTFESMPNPEGRYTFGASCVTACP
YNYLSTDVGSCTLVCPLHNQEVTAEDGTQRCEKCSKPCARVCYGLGMEHLREVRAVTSAN
IQEFAGCKKIFGSLAFLPESFDGDPASNTAPLQPEQLQVFETLEEITGYLYISAWPDSLP
DLSVFQNLQVIRGRILHNGAYSLTLQGLGISWLGLRSLRELGSGLALIHHNTHLCFVHTV
PWDQLFRNPHQALLHTANRPEDECVGEGLACHQLCARGHCWGPGPTQCVNCSQFLRGQEC
VEECRVLQGLPREYVNARHCLPCHPECQPQNGSVTCFGPEADQCVACAHYKDPPFCVARC
PSGVKPDLSYMPIWKFPDEEGACQPCPINCTHSCVDLDDKGCPAEQRASPLTSIISAVVG
ILLVVVLGVVFGILIKRRQQKIRKYTMRRLLQETELVEPLTPSGAMPNQAQMRILKETEL
RKVKVLGSGAFGTVYKGIWIPDGENVKIPVAIKVLRENTSPKANKEILDEAYVMAGVGSP
YVSRLLGICLTSTVQLVTQLMPYGCLLDHVRENRGRLGSQDLLNWCMQIAKGMSYLEDVR
LVHRDLAARNVLVKSPNHVKITDFGLARLLDIDETEYHADGGKVPIKWMALESILRRRFT
HQSDVWSYGVTVWELMTFGAKPYDGIPAREIPDLLEKGERLPQPPICTIDVYMIMVKCWM
IDSECRPRFRELVSEFSRMARDPQRFVVIQNEDLGPASPLDSTFYRSLLEDDDMGDLVDA
EEYLVPQQGFFCPDPAPGAGGMVHHRHRSSSTRSGGGDLTLGLEPSEEEAPRSPLAPSEG
AGSDVFDGDLGMGAAKGLQSLPTHDPSPLQRYSEDPTVPLPSETDGYVAPLTCSPQPEYV
NQPDVRPQPPSPREGPLPAARPAGATLERPKTLSPGKNGVVKDVFAFGGAVENPEYLTPQ
GGAAPQPHPPPAFSPAFDNLYYWDQDPPERGAPPSTFKGTPTAENPEYLGLDVPV
|
|
|
|---|
| BDBM50161957 |
|---|
| n/a |
|---|
| Name | BDBM50161957 |
|---|
| Synonyms: | 4-Dimethylamino-but-2-enoic acid {4-[3-chloro-4-(pyridin-2-ylmethoxy)-phenylamino]-3-cyano-7-ethoxy-quinolin-6-yl}-amide | CHEMBL180022 | HKI-272 | N-(4-(3-chloro-4-(pyridin-2-ylmethoxy)phenylamino)-3-cyano-7-ethoxyquinolin-6-yl)-4-(dimethylamino)but-2-enamide | N-(4-(3-chloro-4-(pyridin-2-ylmethoxy)phenylamino)-3-cyano-7-ethoxyquinolin-6-yl)-4-(dimethylamino)butanamide | NERATINIB | US10822334, Compound Neratinib | US20230382923, Compound Neratinib |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C30H29ClN6O3 |
|---|
| Mol. Mass. | 557.043 |
|---|
| SMILES | CCOc1cc2ncc(C#N)c(Nc3ccc(OCc4ccccn4)c(Cl)c3)c2cc1NC(=O)\C=C\CN(C)C |
|---|
| Structure | 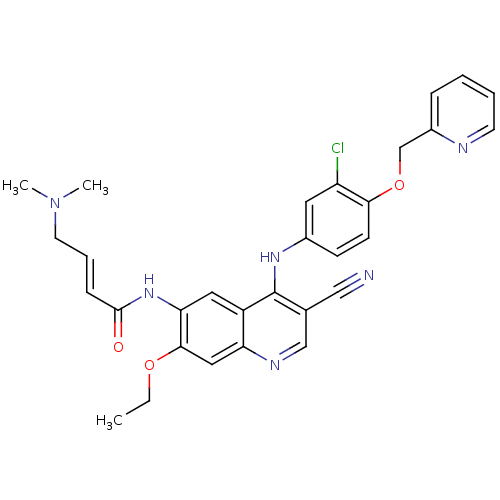
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 McDermott, U; Sharma, SV; Dowell, L; Greninger, P; Montagut, C; Lamb, J; Archibald, H; Raudales, R; Tam, A; Lee, D; Rothenberg, SM; Supko, JG; Sordella, R; Ulkus, LE; Iafrate, AJ; Maheswaran, S; Njauw, CN; Tsao, H; Drew, L; Hanke, JH; Ma, XJ; Erlander, MG; Gray, NS; Haber, DA; Settleman, J Identification of genotype-correlated sensitivity to selective kinase inhibitors by using high-throughput tumor cell line profiling. Proc Natl Acad Sci U S A104:19936-41 (2007) [PubMed] Article
McDermott, U; Sharma, SV; Dowell, L; Greninger, P; Montagut, C; Lamb, J; Archibald, H; Raudales, R; Tam, A; Lee, D; Rothenberg, SM; Supko, JG; Sordella, R; Ulkus, LE; Iafrate, AJ; Maheswaran, S; Njauw, CN; Tsao, H; Drew, L; Hanke, JH; Ma, XJ; Erlander, MG; Gray, NS; Haber, DA; Settleman, J Identification of genotype-correlated sensitivity to selective kinase inhibitors by using high-throughput tumor cell line profiling. Proc Natl Acad Sci U S A104:19936-41 (2007) [PubMed] Article