| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Adenosine receptor A2a |
|---|
| Ligand | BDBM50108022 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_513609 (CHEMBL967913) |
|---|
| Ki | 0.12±n/a nM |
|---|
| Citation |  Mantri, M; de Graaf, O; van Veldhoven, J; Göblyös, A; von Frijtag Drabbe Künzel, JK; Mulder-Krieger, T; Link, R; de Vries, H; Beukers, MW; Brussee, J; Ijzerman, AP 2-Amino-6-furan-2-yl-4-substituted nicotinonitriles as A2A adenosine receptor antagonists. J Med Chem51:4449-55 (2008) [PubMed] Article Mantri, M; de Graaf, O; van Veldhoven, J; Göblyös, A; von Frijtag Drabbe Künzel, JK; Mulder-Krieger, T; Link, R; de Vries, H; Beukers, MW; Brussee, J; Ijzerman, AP 2-Amino-6-furan-2-yl-4-substituted nicotinonitriles as A2A adenosine receptor antagonists. J Med Chem51:4449-55 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Adenosine receptor A2a |
|---|
| Name: | Adenosine receptor A2a |
|---|
| Synonyms: | A2A adenosine receptor (hA2A) | AA2AR_HUMAN | ADENOSINE A2 | ADENOSINE A2a | ADORA2 | ADORA2A | Adenosine A2A receptor (A2AAR) |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 44716.46 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P29274 |
|---|
| Residue: | 412 |
|---|
| Sequence: | MPIMGSSVYITVELAIAVLAILGNVLVCWAVWLNSNLQNVTNYFVVSLAAADIAVGVLAI
PFAITISTGFCAACHGCLFIACFVLVLTQSSIFSLLAIAIDRYIAIRIPLRYNGLVTGTR
AKGIIAICWVLSFAIGLTPMLGWNNCGQPKEGKNHSQGCGEGQVACLFEDVVPMNYMVYF
NFFACVLVPLLLMLGVYLRIFLAARRQLKQMESQPLPGERARSTLQKEVHAAKSLAIIVG
LFALCWLPLHIINCFTFFCPDCSHAPLWLMYLAIVLSHTNSVVNPFIYAYRIREFRQTFR
KIIRSHVLRQQEPFKAAGTSARVLAAHGSDGEQVSLRLNGHPPGVWANGSAPHPERRPNG
YALGLVSGGSAQESQGNTGLPDVELLSHELKGVCPEPPGLDDPLAQDGAGVS
|
|
|
|---|
| BDBM50108022 |
|---|
| n/a |
|---|
| Name | BDBM50108022 |
|---|
| Synonyms: | 4-(2-(5-amino-2-(furan-2-yl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-7-yl)ethyl)-N,N-bis(2-hydroxyethyl)benzenesulfonamide | 4-[2-(5-Amino-2-furan-2-yl-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-7-yl)-ethyl]-N,N-bis-(2-hydroxy-ethyl)-benzenesulfonamide | CHEMBL22717 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H24N8O5S |
|---|
| Mol. Mass. | 512.542 |
|---|
| SMILES | Nc1nc2n(CCc3ccc(cc3)S(=O)(=O)N(CCO)CCO)ncc2c2nc(nn12)-c1ccco1 |
|---|
| Structure | 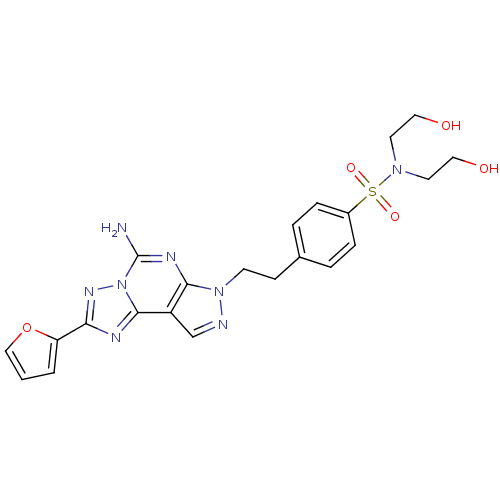
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Mantri, M; de Graaf, O; van Veldhoven, J; Göblyös, A; von Frijtag Drabbe Künzel, JK; Mulder-Krieger, T; Link, R; de Vries, H; Beukers, MW; Brussee, J; Ijzerman, AP 2-Amino-6-furan-2-yl-4-substituted nicotinonitriles as A2A adenosine receptor antagonists. J Med Chem51:4449-55 (2008) [PubMed] Article
Mantri, M; de Graaf, O; van Veldhoven, J; Göblyös, A; von Frijtag Drabbe Künzel, JK; Mulder-Krieger, T; Link, R; de Vries, H; Beukers, MW; Brussee, J; Ijzerman, AP 2-Amino-6-furan-2-yl-4-substituted nicotinonitriles as A2A adenosine receptor antagonists. J Med Chem51:4449-55 (2008) [PubMed] Article