| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cannabinoid receptor 1 |
|---|
| Ligand | BDBM50272428 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_510479 (CHEMBL1006420) |
|---|
| EC50 | 990±n/a nM |
|---|
| Citation |  Cheng, Y; Albrecht, BK; Brown, J; Buchanan, JL; Buckner, WH; DiMauro, EF; Emkey, R; Fremeau, RT; Harmange, JC; Hoffman, BJ; Huang, L; Huang, M; Lee, JH; Lin, FF; Martin, MW; Nguyen, HQ; Patel, VF; Tomlinson, SA; White, RD; Xia, X; Hitchcock, SA Discovery and optimization of a novel series of N-arylamide oxadiazoles as potent, highly selective and orally bioavailable cannabinoid receptor 2 (CB2) agonists. J Med Chem51:5019-34 (2008) [PubMed] Article Cheng, Y; Albrecht, BK; Brown, J; Buchanan, JL; Buckner, WH; DiMauro, EF; Emkey, R; Fremeau, RT; Harmange, JC; Hoffman, BJ; Huang, L; Huang, M; Lee, JH; Lin, FF; Martin, MW; Nguyen, HQ; Patel, VF; Tomlinson, SA; White, RD; Xia, X; Hitchcock, SA Discovery and optimization of a novel series of N-arylamide oxadiazoles as potent, highly selective and orally bioavailable cannabinoid receptor 2 (CB2) agonists. J Med Chem51:5019-34 (2008) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cannabinoid receptor 1 |
|---|
| Name: | Cannabinoid receptor 1 |
|---|
| Synonyms: | CANN6 | CANNABINOID CB1 | CB-R | CB1 | CNR | CNR1 | CNR1_HUMAN | Cannabinoid CB1 receptor | Cannabinoid receptor | Cannabinoid receptor 1 (CB1) | Cannabinoid receptor 1 (brain) |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 52868.96 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P21554 |
|---|
| Residue: | 472 |
|---|
| Sequence: | MKSILDGLADTTFRTITTDLLYVGSNDIQYEDIKGDMASKLGYFPQKFPLTSFRGSPFQE
KMTAGDNPQLVPADQVNITEFYNKSLSSFKENEENIQCGENFMDIECFMVLNPSQQLAIA
VLSLTLGTFTVLENLLVLCVILHSRSLRCRPSYHFIGSLAVADLLGSVIFVYSFIDFHVF
HRKDSRNVFLFKLGGVTASFTASVGSLFLTAIDRYISIHRPLAYKRIVTRPKAVVAFCLM
WTIAIVIAVLPLLGWNCEKLQSVCSDIFPHIDETYLMFWIGVTSVLLLFIVYAYMYILWK
AHSHAVRMIQRGTQKSIIIHTSEDGKVQVTRPDQARMDIRLAKTLVLILVVLIICWGPLL
AIMVYDVFGKMNKLIKTVFAFCSMLCLLNSTVNPIIYALRSKDLRHAFRSMFPSCEGTAQ
PLDNSMGDSDCLHKHANNAASVHRAAESCIKSTVKIAKVTMSVSTDTSAEAL
|
|
|
|---|
| BDBM50272428 |
|---|
| n/a |
|---|
| Name | BDBM50272428 |
|---|
| Synonyms: | 6-Chloro-3-(3-(3-(2-chloro-4-fluorophenyl)-1,2,4-oxadiazol-5-yl)propoxy)quinoline | CHEMBL498469 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H14Cl2FN3O2 |
|---|
| Mol. Mass. | 418.248 |
|---|
| SMILES | Fc1ccc(-c2noc(CCCOc3cnc4ccc(Cl)cc4c3)n2)c(Cl)c1 |
|---|
| Structure | 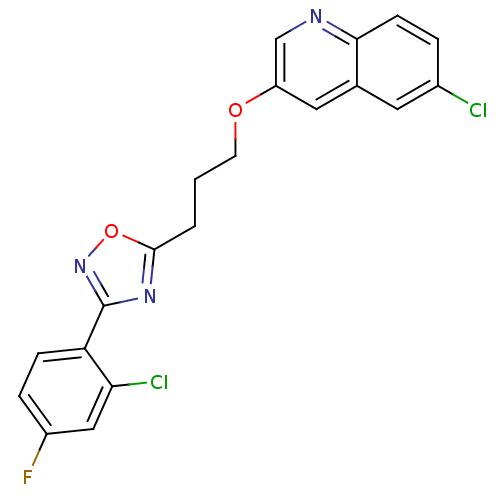
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Cheng, Y; Albrecht, BK; Brown, J; Buchanan, JL; Buckner, WH; DiMauro, EF; Emkey, R; Fremeau, RT; Harmange, JC; Hoffman, BJ; Huang, L; Huang, M; Lee, JH; Lin, FF; Martin, MW; Nguyen, HQ; Patel, VF; Tomlinson, SA; White, RD; Xia, X; Hitchcock, SA Discovery and optimization of a novel series of N-arylamide oxadiazoles as potent, highly selective and orally bioavailable cannabinoid receptor 2 (CB2) agonists. J Med Chem51:5019-34 (2008) [PubMed] Article
Cheng, Y; Albrecht, BK; Brown, J; Buchanan, JL; Buckner, WH; DiMauro, EF; Emkey, R; Fremeau, RT; Harmange, JC; Hoffman, BJ; Huang, L; Huang, M; Lee, JH; Lin, FF; Martin, MW; Nguyen, HQ; Patel, VF; Tomlinson, SA; White, RD; Xia, X; Hitchcock, SA Discovery and optimization of a novel series of N-arylamide oxadiazoles as potent, highly selective and orally bioavailable cannabinoid receptor 2 (CB2) agonists. J Med Chem51:5019-34 (2008) [PubMed] Article