| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Purine nucleoside phosphorylase |
|---|
| Ligand | BDBM50293090 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_519471 (CHEMBL951777) |
|---|
| Ki | 0.003±n/a nM |
|---|
| Citation |  Clinch, K; Evans, GB; Fröhlich, RF; Furneaux, RH; Kelly, PM; Legentil, L; Murkin, AS; Li, L; Schramm, VL; Tyler, PC; Woolhouse, AD Third-generation immucillins: syntheses and bioactivities of acyclic immucillin inhibitors of human purine nucleoside phosphorylase. J Med Chem52:1126-43 (2009) [PubMed] Article Clinch, K; Evans, GB; Fröhlich, RF; Furneaux, RH; Kelly, PM; Legentil, L; Murkin, AS; Li, L; Schramm, VL; Tyler, PC; Woolhouse, AD Third-generation immucillins: syntheses and bioactivities of acyclic immucillin inhibitors of human purine nucleoside phosphorylase. J Med Chem52:1126-43 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Purine nucleoside phosphorylase |
|---|
| Name: | Purine nucleoside phosphorylase |
|---|
| Synonyms: | Inosine phosphorylase | Inosine-guanosine phosphorylase | NP | PNP | PNPH_HUMAN | Purine nucleoside phosphorylase (PNPase) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 32119.53 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | n/a |
|---|
| Residue: | 289 |
|---|
| Sequence: | MENGYTYEDYKNTAEWLLSHTKHRPQVAIICGSGLGGLTDKLTQAQIFDYGEIPNFPRST
VPGHAGRLVFGFLNGRACVMMQGRFHMYEGYPLWKVTFPVRVFHLLGVDTLVVTNAAGGL
NPKFEVGDIMLIRDHINLPGFSGQNPLRGPNDERFGDRFPAMSDAYDRTMRQRALSTWKQ
MGEQRELQEGTYVMVAGPSFETVAECRVLQKLGADAVGMSTVPEVIVARHCGLRVFGFSL
ITNKVIMDYESLEKANHEEVLAAGKQAAQKLEQFVSILMASIPLPDKAS
|
|
|
|---|
| BDBM50293090 |
|---|
| n/a |
|---|
| Name | BDBM50293090 |
|---|
| Synonyms: | 2-Amino-7-({[(2R,3S)-1,3,4-trihydroxybutan-2-yl]amino}methyl)-3,5-dihydro-4H-pyrrolo[3,2-d]pyrimidin-4-one | CHEMBL516050 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C11H17N5O4 |
|---|
| Mol. Mass. | 283.2838 |
|---|
| SMILES | Nc1nc2c(CN[C@H](CO)[C@H](O)CO)c[nH]c2c(=O)[nH]1 |r| |
|---|
| Structure | 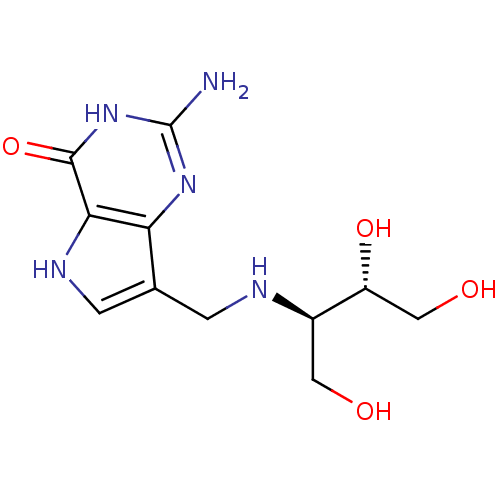
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Clinch, K; Evans, GB; Fröhlich, RF; Furneaux, RH; Kelly, PM; Legentil, L; Murkin, AS; Li, L; Schramm, VL; Tyler, PC; Woolhouse, AD Third-generation immucillins: syntheses and bioactivities of acyclic immucillin inhibitors of human purine nucleoside phosphorylase. J Med Chem52:1126-43 (2009) [PubMed] Article
Clinch, K; Evans, GB; Fröhlich, RF; Furneaux, RH; Kelly, PM; Legentil, L; Murkin, AS; Li, L; Schramm, VL; Tyler, PC; Woolhouse, AD Third-generation immucillins: syntheses and bioactivities of acyclic immucillin inhibitors of human purine nucleoside phosphorylase. J Med Chem52:1126-43 (2009) [PubMed] Article