| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Vascular endothelial growth factor receptor 2 |
|---|
| Ligand | BDBM50277584 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_501694 (CHEMBL981442) |
|---|
| IC50 | 36±n/a nM |
|---|
| Citation |  Pollard, JR; Mortimore, M Discovery and development of aurora kinase inhibitors as anticancer agents. J Med Chem52:2629-51 (2009) [PubMed] Article Pollard, JR; Mortimore, M Discovery and development of aurora kinase inhibitors as anticancer agents. J Med Chem52:2629-51 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Vascular endothelial growth factor receptor 2 |
|---|
| Name: | Vascular endothelial growth factor receptor 2 |
|---|
| Synonyms: | CD_antigen=CD309 | FLK1 | Fetal liver kinase 1 (FLK-1) | Flk-1/KDR | KDR | Kinase Insert Domain Receptor | Protein-tyrosine kinase receptor Flk-1 | VEGFR kinase (KDR) | VEGFR-2 | VEGFR-2 (KDR) | VEGFR2 | VGFR2_HUMAN | Vascular Endothelial Growth Factor Receptor Kinase 2 | Vascular endothelial growth factor receptor (VEGFR-2) | Vascular endothelial growth factor receptor 2 (KDR) | Vascular endothelial growth factor receptor 2 (VEGFR-2) | Vascular endothelial growth factor receptor 2 (VEGFR2) | Vascular endothelial growth factor receptor 2 precursor (VEGFR-2) | Vascular endothelial growth factor receptor-2 (VEGFR-2) |
|---|
| Type: | Receptor Tyrosine Kinase |
|---|
| Mol. Mass.: | 151510.97 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P35968 |
|---|
| Residue: | 1356 |
|---|
| Sequence: | MQSKVLLAVALWLCVETRAASVGLPSVSLDLPRLSIQKDILTIKANTTLQITCRGQRDLD
WLWPNNQSGSEQRVEVTECSDGLFCKTLTIPKVIGNDTGAYKCFYRETDLASVIYVYVQD
YRSPFIASVSDQHGVVYITENKNKTVVIPCLGSISNLNVSLCARYPEKRFVPDGNRISWD
SKKGFTIPSYMISYAGMVFCEAKINDESYQSIMYIVVVVGYRIYDVVLSPSHGIELSVGE
KLVLNCTARTELNVGIDFNWEYPSSKHQHKKLVNRDLKTQSGSEMKKFLSTLTIDGVTRS
DQGLYTCAASSGLMTKKNSTFVRVHEKPFVAFGSGMESLVEATVGERVRIPAKYLGYPPP
EIKWYKNGIPLESNHTIKAGHVLTIMEVSERDTGNYTVILTNPISKEKQSHVVSLVVYVP
PQIGEKSLISPVDSYQYGTTQTLTCTVYAIPPPHHIHWYWQLEEECANEPSQAVSVTNPY
PCEEWRSVEDFQGGNKIEVNKNQFALIEGKNKTVSTLVIQAANVSALYKCEAVNKVGRGE
RVISFHVTRGPEITLQPDMQPTEQESVSLWCTADRSTFENLTWYKLGPQPLPIHVGELPT
PVCKNLDTLWKLNATMFSNSTNDILIMELKNASLQDQGDYVCLAQDRKTKKRHCVVRQLT
VLERVAPTITGNLENQTTSIGESIEVSCTASGNPPPQIMWFKDNETLVEDSGIVLKDGNR
NLTIRRVRKEDEGLYTCQACSVLGCAKVEAFFIIEGAQEKTNLEIIILVGTAVIAMFFWL
LLVIILRTVKRANGGELKTGYLSIVMDPDELPLDEHCERLPYDASKWEFPRDRLKLGKPL
GRGAFGQVIEADAFGIDKTATCRTVAVKMLKEGATHSEHRALMSELKILIHIGHHLNVVN
LLGACTKPGGPLMVIVEFCKFGNLSTYLRSKRNEFVPYKTKGARFRQGKDYVGAIPVDLK
RRLDSITSSQSSASSGFVEEKSLSDVEEEEAPEDLYKDFLTLEHLICYSFQVAKGMEFLA
SRKCIHRDLAARNILLSEKNVVKICDFGLARDIYKDPDYVRKGDARLPLKWMAPETIFDR
VYTIQSDVWSFGVLLWEIFSLGASPYPGVKIDEEFCRRLKEGTRMRAPDYTTPEMYQTML
DCWHGEPSQRPTFSELVEHLGNLLQANAQQDGKDYIVLPISETLSMEEDSGLSLPTSPVS
CMEEEEVCDPKFHYDNTAGISQYLQNSKRKSRPVSVKTFEDIPLEEPEVKVIPDDNQTDS
GMVLASEELKTLEDRTKLSPSFGGMVPSKSRESVASEGSNQTSGYQSGYHSDDTDTTVYS
SEEAELLKLIEIGVQTGSTAQILQPDSGTTLSSPPV
|
|
|
|---|
| BDBM50277584 |
|---|
| n/a |
|---|
| Name | BDBM50277584 |
|---|
| Synonyms: | CHEMBL482968 | N-(5-methyl-1H-pyrazol-3-yl)-6-(4-methylpiperazin-1-yl)-2-styrylpyrimidin-4-amine |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H25N7 |
|---|
| Mol. Mass. | 375.4701 |
|---|
| SMILES | CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(C=Cc2ccccc2)n1 |w:19.20| |
|---|
| Structure | 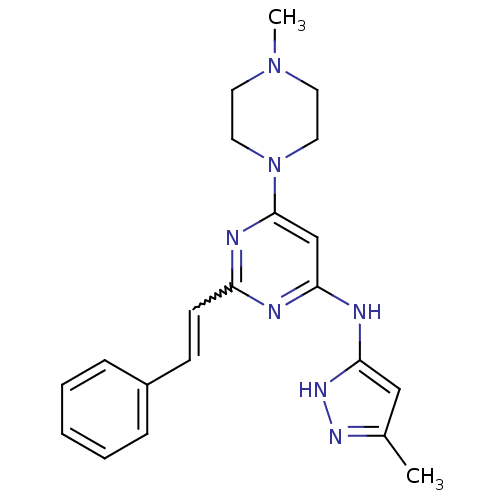
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Pollard, JR; Mortimore, M Discovery and development of aurora kinase inhibitors as anticancer agents. J Med Chem52:2629-51 (2009) [PubMed] Article
Pollard, JR; Mortimore, M Discovery and development of aurora kinase inhibitors as anticancer agents. J Med Chem52:2629-51 (2009) [PubMed] Article