| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Mu-type opioid receptor |
|---|
| Ligand | BDBM50295072 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_573428 (CHEMBL1061323) |
|---|
| EC50 | 18±n/a nM |
|---|
| Citation |  Yamamoto, T; Nair, P; Jacobsen, NE; Vagner, J; Kulkarni, V; Davis, P; Ma, SW; Navratilova, E; Yamamura, HI; Vanderah, TW; Porreca, F; Lai, J; Hruby, VJ Improving metabolic stability by glycosylation: bifunctional peptide derivatives that are opioid receptor agonists and neurokinin 1 receptor antagonists. J Med Chem52:5164-75 (2010) [PubMed] Article Yamamoto, T; Nair, P; Jacobsen, NE; Vagner, J; Kulkarni, V; Davis, P; Ma, SW; Navratilova, E; Yamamura, HI; Vanderah, TW; Porreca, F; Lai, J; Hruby, VJ Improving metabolic stability by glycosylation: bifunctional peptide derivatives that are opioid receptor agonists and neurokinin 1 receptor antagonists. J Med Chem52:5164-75 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Mu-type opioid receptor |
|---|
| Name: | Mu-type opioid receptor |
|---|
| Synonyms: | MOR-1 | MUOR1 | Mu-type opioid receptor (MOR) | OPIATE Mu | OPRM_RAT | Opiate non-selective | Opioid receptor B | Oprm1 | Ror-b |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 44503.11 |
|---|
| Organism: | Rattus norvegicus (rat) |
|---|
| Description: | Competition binding assays were carried out using membrane preparations from transfected HN9.10 cells that constitutively expressed the mu opioid receptor. |
|---|
| Residue: | 398 |
|---|
| Sequence: | MDSSTGPGNTSDCSDPLAQASCSPAPGSWLNLSHVDGNQSDPCGLNRTGLGGNDSLCPQT
GSPSMVTAITIMALYSIVCVVGLFGNFLVMYVIVRYTKMKTATNIYIFNLALADALATST
LPFQSVNYLMGTWPFGTILCKIVISIDYYNMFTSIFTLCTMSVDRYIAVCHPVKALDFRT
PRNAKIVNVCNWILSSAIGLPVMFMATTKYRQGSIDCTLTFSHPTWYWENLLKICVFIFA
FIMPVLIITVCYGLMILRLKSVRMLSGSKEKDRNLRRITRMVLVVVAVFIVCWTPIHIYV
IIKALITIPETTFQTVSWHFCIALGYTNSCLNPVLYAFLDENFKRCFREFCIPTSSTIEQ
QNSTRVRQNTREHPSTANTVDRTNHQLENLEAETAPLP
|
|
|
|---|
| BDBM50295072 |
|---|
| n/a |
|---|
| Name | BDBM50295072 |
|---|
| Synonyms: | (S)-1-((2S,5S,11R,14S)-14-amino-5-benzyl-2-butyl-15-(4-hydroxyphenyl)-11-methyl-4,7,10,13-tetraoxo-3,6,9,12-tetraazapentadecane)-N-((S)-1-((S)-1-(3,5-bis(trifluoromethyl)benzylamino)-3-(1H-indol-3-yl)-1-oxopropan-2-ylamino)-1-oxo-3-((2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yloxy)propan-2-yl)pyrrolidine-2-carboxamide | CHEMBL539193 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C63H76F6N10O15 |
|---|
| Mol. Mass. | 1327.326 |
|---|
| SMILES | CCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| |
|---|
| Structure | 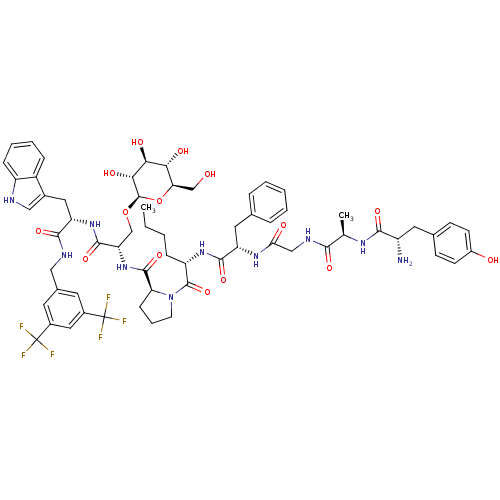
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Yamamoto, T; Nair, P; Jacobsen, NE; Vagner, J; Kulkarni, V; Davis, P; Ma, SW; Navratilova, E; Yamamura, HI; Vanderah, TW; Porreca, F; Lai, J; Hruby, VJ Improving metabolic stability by glycosylation: bifunctional peptide derivatives that are opioid receptor agonists and neurokinin 1 receptor antagonists. J Med Chem52:5164-75 (2010) [PubMed] Article
Yamamoto, T; Nair, P; Jacobsen, NE; Vagner, J; Kulkarni, V; Davis, P; Ma, SW; Navratilova, E; Yamamura, HI; Vanderah, TW; Porreca, F; Lai, J; Hruby, VJ Improving metabolic stability by glycosylation: bifunctional peptide derivatives that are opioid receptor agonists and neurokinin 1 receptor antagonists. J Med Chem52:5164-75 (2010) [PubMed] Article