| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cannabinoid receptor 2 |
|---|
| Ligand | BDBM50298941 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_588142 (CHEMBL1049259) |
|---|
| Ki | 2.4±n/a nM |
|---|
| Citation |  Silvestri, R; Ligresti, A; La Regina, G; Piscitelli, F; Gatti, V; Brizzi, A; Pasquini, S; Lavecchia, A; Allarà, M; Fantini, N; Carai, MA; Novellino, E; Colombo, G; Di Marzo, V; Corelli, F Synthesis, cannabinoid receptor affinity, molecular modeling studies and in vivo pharmacological evaluation of new substituted 1-aryl-5-(1H-pyrrol-1-yl)-1H-pyrazole-3-carboxamides. 2. Effect of the 3-carboxamide substituent on the affinity and selectivity profile. Bioorg Med Chem17:5549-64 (2009) [PubMed] Article Silvestri, R; Ligresti, A; La Regina, G; Piscitelli, F; Gatti, V; Brizzi, A; Pasquini, S; Lavecchia, A; Allarà, M; Fantini, N; Carai, MA; Novellino, E; Colombo, G; Di Marzo, V; Corelli, F Synthesis, cannabinoid receptor affinity, molecular modeling studies and in vivo pharmacological evaluation of new substituted 1-aryl-5-(1H-pyrrol-1-yl)-1H-pyrazole-3-carboxamides. 2. Effect of the 3-carboxamide substituent on the affinity and selectivity profile. Bioorg Med Chem17:5549-64 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cannabinoid receptor 2 |
|---|
| Name: | Cannabinoid receptor 2 |
|---|
| Synonyms: | CANNABINOID CB2 | CB-2 | CB2 | CB2A | CB2B | CNR2 | CNR2_HUMAN | CX5 | Cannabinoid CB2 receptor | Cannabinoid receptor 2 (CB2) | Cannabinoid receptor 2 (CB2R) | hCB2 |
|---|
| Type: | G Protein-Coupled Receptor (GPCR) |
|---|
| Mol. Mass.: | 39690.94 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P34972 |
|---|
| Residue: | 360 |
|---|
| Sequence: | MEECWVTEIANGSKDGLDSNPMKDYMILSGPQKTAVAVLCTLLGLLSALENVAVLYLILS
SHQLRRKPSYLFIGSLAGADFLASVVFACSFVNFHVFHGVDSKAVFLLKIGSVTMTFTAS
VGSLLLTAIDRYLCLRYPPSYKALLTRGRALVTLGIMWVLSALVSYLPLMGWTCCPRPCS
ELFPLIPNDYLLSWLLFIAFLFSGIIYTYGHVLWKAHQHVASLSGHQDRQVPGMARMRLD
VRLAKTLGLVLAVLLICWFPVLALMAHSLATTLSDQVKKAFAFCSMLCLINSMVNPVIYA
LRSGEIRSSAHHCLAHWKKCVRGLGSEAKEEAPRSSVTETEADGKITPWPDSRDLDLSDC
|
|
|
|---|
| BDBM50298941 |
|---|
| n/a |
|---|
| Name | BDBM50298941 |
|---|
| Synonyms: | (S)-N-[1-(1-Cyclohexyl)ethyl]1-(3,4-dichlorophenyl)-4-methyl-5-(1H-pyrrol-1-yl)-1H-pyrazole-3-carboxamide | CHEMBL573546 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C23H26Cl2N4O |
|---|
| Mol. Mass. | 445.385 |
|---|
| SMILES | C[C@H](NC(=O)c1nn(c(c1C)-n1cccc1)-c1ccc(Cl)c(Cl)c1)C1CCCCC1 |r| |
|---|
| Structure | 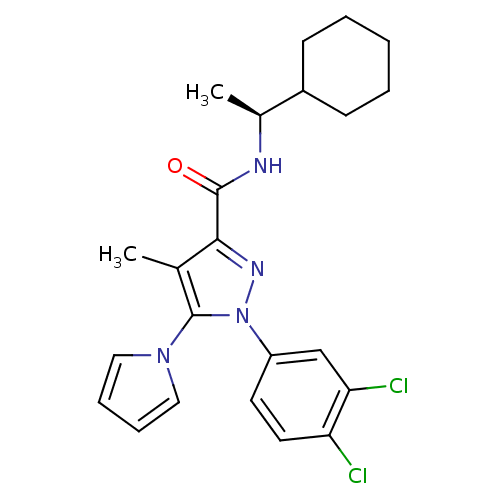
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Silvestri, R; Ligresti, A; La Regina, G; Piscitelli, F; Gatti, V; Brizzi, A; Pasquini, S; Lavecchia, A; Allarà, M; Fantini, N; Carai, MA; Novellino, E; Colombo, G; Di Marzo, V; Corelli, F Synthesis, cannabinoid receptor affinity, molecular modeling studies and in vivo pharmacological evaluation of new substituted 1-aryl-5-(1H-pyrrol-1-yl)-1H-pyrazole-3-carboxamides. 2. Effect of the 3-carboxamide substituent on the affinity and selectivity profile. Bioorg Med Chem17:5549-64 (2009) [PubMed] Article
Silvestri, R; Ligresti, A; La Regina, G; Piscitelli, F; Gatti, V; Brizzi, A; Pasquini, S; Lavecchia, A; Allarà, M; Fantini, N; Carai, MA; Novellino, E; Colombo, G; Di Marzo, V; Corelli, F Synthesis, cannabinoid receptor affinity, molecular modeling studies and in vivo pharmacological evaluation of new substituted 1-aryl-5-(1H-pyrrol-1-yl)-1H-pyrazole-3-carboxamides. 2. Effect of the 3-carboxamide substituent on the affinity and selectivity profile. Bioorg Med Chem17:5549-64 (2009) [PubMed] Article