| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Carbonic anhydrase 3 |
|---|
| Ligand | BDBM50300204 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_592885 (CHEMBL1046630) |
|---|
| Ki | 374±n/a nM |
|---|
| Citation |  Temperini, C; Innocenti, A; Scozzafava, A; Parkkila, S; Supuran, CT The coumarin-binding site in carbonic anhydrase accommodates structurally diverse inhibitors: the antiepileptic lacosamide as an example and lead molecule for novel classes of carbonic anhydrase inhibitors. J Med Chem53:850-4 (2010) [PubMed] Article Temperini, C; Innocenti, A; Scozzafava, A; Parkkila, S; Supuran, CT The coumarin-binding site in carbonic anhydrase accommodates structurally diverse inhibitors: the antiepileptic lacosamide as an example and lead molecule for novel classes of carbonic anhydrase inhibitors. J Med Chem53:850-4 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Carbonic anhydrase 3 |
|---|
| Name: | Carbonic anhydrase 3 |
|---|
| Synonyms: | CA-III | CA3 | CAH3_HUMAN | Carbonate dehydratase III | Carbonic Anhydrase III | Carbonic anhydrase | Carbonic anhydrase 3 (CA III) | Carbonic anhydrase III (CA III) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 29562.11 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Human cloned isozyme. |
|---|
| Residue: | 260 |
|---|
| Sequence: | MAKEWGYASHNGPDHWHELFPNAKGENQSPVELHTKDIRHDPSLQPWSVSYDGGSAKTIL
NNGKTCRVVFDDTYDRSMLRGGPLPGPYRLRQFHLHWGSSDDHGSEHTVDGVKYAAELHL
VHWNPKYNTFKEALKQRDGIAVIGIFLKIGHENGEFQIFLDALDKIKTKGKEAPFTKFDP
SCLFPACRDYWTYQGSFTTPPCEECIVWLLLKEPMTVSSDQMAKLRSLLSSAENEPPVPL
VSNWRPPQPINNRVVRASFK
|
|
|
|---|
| BDBM50300204 |
|---|
| n/a |
|---|
| Name | BDBM50300204 |
|---|
| Synonyms: | (2R)-2-acetylamino-N-benzyl-3-methoxypropanamide | (R)-2-Acetylamino-N-benzyl-3-methoxy-propionamide | (R)-2-acetamido-N-benzyl-3-methoxypropanamide | (R)-N-benzyl 2-acetamido-3-methoxypropionamide | (R)-N-benzyl 2-acetamido-3-methoxypropionamide, | CHEMBL58323 | Erlosamide | LACOSAMIDE |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C13H18N2O3 |
|---|
| Mol. Mass. | 250.2936 |
|---|
| SMILES | COC[C@@H](NC(C)=O)C(=O)NCc1ccccc1 |r| |
|---|
| Structure | 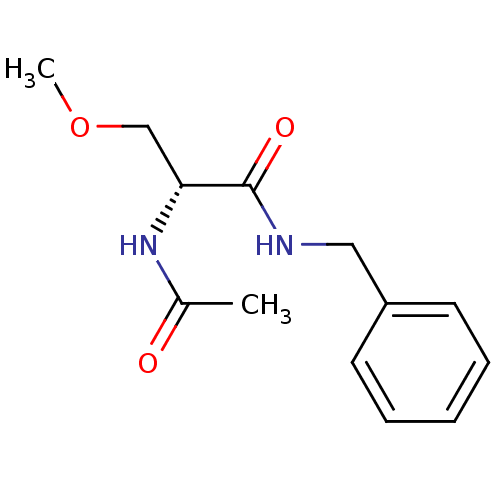
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Temperini, C; Innocenti, A; Scozzafava, A; Parkkila, S; Supuran, CT The coumarin-binding site in carbonic anhydrase accommodates structurally diverse inhibitors: the antiepileptic lacosamide as an example and lead molecule for novel classes of carbonic anhydrase inhibitors. J Med Chem53:850-4 (2010) [PubMed] Article
Temperini, C; Innocenti, A; Scozzafava, A; Parkkila, S; Supuran, CT The coumarin-binding site in carbonic anhydrase accommodates structurally diverse inhibitors: the antiepileptic lacosamide as an example and lead molecule for novel classes of carbonic anhydrase inhibitors. J Med Chem53:850-4 (2010) [PubMed] Article