| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Beta-carbonic anhydrase 1 |
|---|
| Ligand | BDBM10872 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_599731 (CHEMBL1048175) |
|---|
| Ki | 853±n/a nM |
|---|
| Citation |  Maresca, A; Carta, F; Vullo, D; Scozzafava, A; Supuran, CT Carbonic anhydrase inhibitors. Inhibition of the Rv1284 and Rv3273 beta-carbonic anhydrases from Mycobacterium tuberculosis with diazenylbenzenesulfonamides. Bioorg Med Chem Lett19:4929-32 (2009) [PubMed] Article Maresca, A; Carta, F; Vullo, D; Scozzafava, A; Supuran, CT Carbonic anhydrase inhibitors. Inhibition of the Rv1284 and Rv3273 beta-carbonic anhydrases from Mycobacterium tuberculosis with diazenylbenzenesulfonamides. Bioorg Med Chem Lett19:4929-32 (2009) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Beta-carbonic anhydrase 1 |
|---|
| Name: | Beta-carbonic anhydrase 1 |
|---|
| Synonyms: | β-Carbonic anhydrase 1 (CA 1) | Carbonic Anhydrase (mtCA 1) | MTCA1_MYCTU | Uncharacterized protein Rv1284/MT1322 | canA | mtcA1 |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 18186.06 |
|---|
| Organism: | Mycobacterium tuberculosis |
|---|
| Description: | The recombinant GST-mtCA1 construct was cloned, expressed, and further purified from E. coli. The purified protein was used in inhibition assays. |
|---|
| Residue: | 163 |
|---|
| Sequence: | MTVTDDYLANNVDYASGFKGPLPMPPSKHIAIVACMDARLDVYRMLGIKEGEAHVIRNAG
CVVTDDVIRSLAISQRLLGTREIILLHHTDCGMLTFTDDDFKRAIQDETGIRPTWSPESY
PDAVEDVRQSLRRIEVNPFVTKHTSLRGFVFDVATGKLNEVTP
|
|
|
|---|
| BDBM10872 |
|---|
| n/a |
|---|
| Name | BDBM10872 |
|---|
| Synonyms: | 4-amino-N-[2-(4-sulfamoylphenyl)ethyl]benzene-1-sulfonamide | CHEMBL7146 | aromatic/heteroaromatic sulfonamide 17 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C14H17N3O4S2 |
|---|
| Mol. Mass. | 355.432 |
|---|
| SMILES | Nc1ccc(cc1)S(=O)(=O)NCCc1ccc(cc1)S(N)(=O)=O |
|---|
| Structure | 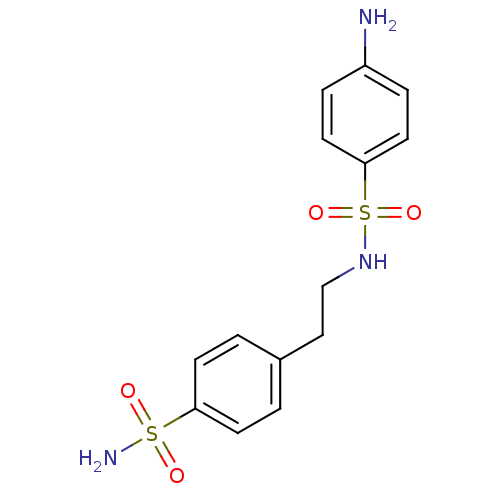
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Maresca, A; Carta, F; Vullo, D; Scozzafava, A; Supuran, CT Carbonic anhydrase inhibitors. Inhibition of the Rv1284 and Rv3273 beta-carbonic anhydrases from Mycobacterium tuberculosis with diazenylbenzenesulfonamides. Bioorg Med Chem Lett19:4929-32 (2009) [PubMed] Article
Maresca, A; Carta, F; Vullo, D; Scozzafava, A; Supuran, CT Carbonic anhydrase inhibitors. Inhibition of the Rv1284 and Rv3273 beta-carbonic anhydrases from Mycobacterium tuberculosis with diazenylbenzenesulfonamides. Bioorg Med Chem Lett19:4929-32 (2009) [PubMed] Article