| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2A6 |
|---|
| Ligand | BDBM50318596 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_633185 (CHEMBL1118622) |
|---|
| IC50 | >10000±n/a nM |
|---|
| Citation |  Bavetsias, V; Large, JM; Sun, C; Bouloc, N; Kosmopoulou, M; Matteucci, M; Wilsher, NE; Martins, V; Reynisson, J; Atrash, B; Faisal, A; Urban, F; Valenti, M; de Haven Brandon, A; Box, G; Raynaud, FI; Workman, P; Eccles, SA; Bayliss, R; Blagg, J; Linardopoulos, S; McDonald, E Imidazo[4,5-b]pyridine derivatives as inhibitors of Aurora kinases: lead optimization studies toward the identification of an orally bioavailable preclinical development candidate. J Med Chem53:5213-28 (2010) [PubMed] Article Bavetsias, V; Large, JM; Sun, C; Bouloc, N; Kosmopoulou, M; Matteucci, M; Wilsher, NE; Martins, V; Reynisson, J; Atrash, B; Faisal, A; Urban, F; Valenti, M; de Haven Brandon, A; Box, G; Raynaud, FI; Workman, P; Eccles, SA; Bayliss, R; Blagg, J; Linardopoulos, S; McDonald, E Imidazo[4,5-b]pyridine derivatives as inhibitors of Aurora kinases: lead optimization studies toward the identification of an orally bioavailable preclinical development candidate. J Med Chem53:5213-28 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2A6 |
|---|
| Name: | Cytochrome P450 2A6 |
|---|
| Synonyms: | 1,4-cineole 2-exo-monooxygenase | 1.14.13.- | CP2A6_HUMAN | CYP2A3 | CYP2A6 | CYPIIA6 | Coumarin 7-hydroxylase | Cytochrome P450 2A6 | Cytochrome P450 IIA3 | Cytochrome P450(I) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 56514.34 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P11509 |
|---|
| Residue: | 494 |
|---|
| Sequence: | MLASGMLLVALLVCLTVMVLMSVWQQRKSKGKLPPGPTPLPFIGNYLQLNTEQMYNSLMK
ISERYGPVFTIHLGPRRVVVLCGHDAVREALVDQAEEFSGRGEQATFDWVFKGYGVVFSN
GERAKQLRRFSIATLRDFGVGKRGIEERIQEEAGFLIDALRGTGGANIDPTFFLSRTVSN
VISSIVFGDRFDYKDKEFLSLLRMMLGIFQFTSTSTGQLYEMFSSVMKHLPGPQQQAFQL
LQGLEDFIAKKVEHNQRTLDPNSPRDFIDSFLIRMQEEEKNPNTEFYLKNLVMTTLNLFI
GGTETVSTTLRYGFLLLMKHPEVEAKVHEEIDRVIGKNRQPKFEDRAKMPYMEAVIHEIQ
RFGDVIPMSLARRVKKDTKFRDFFLPKGTEVYPMLGSVLRDPSFFSNPQDFNPQHFLNEK
GQFKKSDAFVPFSIGKRNCFGEGLARMELFLFFTTVMQNFRLKSSQSPKDIDVSPKHVGF
ATIPRNYTMSFLPR
|
|
|
|---|
| BDBM50318596 |
|---|
| n/a |
|---|
| Name | BDBM50318596 |
|---|
| Synonyms: | 3-((4-(6-Bromo-2-(4-(4-methylpiperazin-1-yl)phenyl)-3H-imidazo-[4,5-b]pyridin-7-yl)piperazin-1-yl)methyl)-5-methylisoxazole | CHEMBL1086579 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H27BrN8O |
|---|
| Mol. Mass. | 475.385 |
|---|
| SMILES | CN1CCN(CC1)c1nc2ncc(Br)c(N3CCN(Cc4cc(C)on4)CC3)c2[nH]1 |
|---|
| Structure | 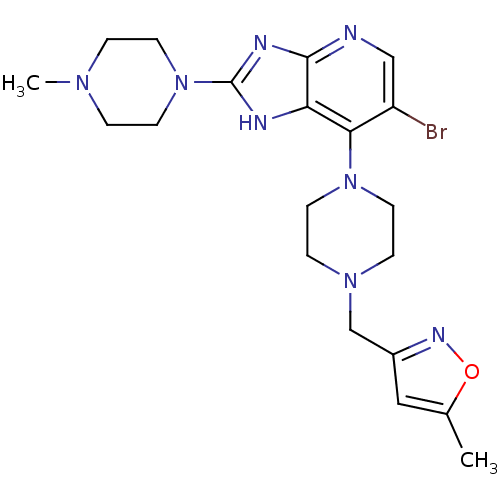
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Bavetsias, V; Large, JM; Sun, C; Bouloc, N; Kosmopoulou, M; Matteucci, M; Wilsher, NE; Martins, V; Reynisson, J; Atrash, B; Faisal, A; Urban, F; Valenti, M; de Haven Brandon, A; Box, G; Raynaud, FI; Workman, P; Eccles, SA; Bayliss, R; Blagg, J; Linardopoulos, S; McDonald, E Imidazo[4,5-b]pyridine derivatives as inhibitors of Aurora kinases: lead optimization studies toward the identification of an orally bioavailable preclinical development candidate. J Med Chem53:5213-28 (2010) [PubMed] Article
Bavetsias, V; Large, JM; Sun, C; Bouloc, N; Kosmopoulou, M; Matteucci, M; Wilsher, NE; Martins, V; Reynisson, J; Atrash, B; Faisal, A; Urban, F; Valenti, M; de Haven Brandon, A; Box, G; Raynaud, FI; Workman, P; Eccles, SA; Bayliss, R; Blagg, J; Linardopoulos, S; McDonald, E Imidazo[4,5-b]pyridine derivatives as inhibitors of Aurora kinases: lead optimization studies toward the identification of an orally bioavailable preclinical development candidate. J Med Chem53:5213-28 (2010) [PubMed] Article