| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Sialidase-3 |
|---|
| Ligand | BDBM50331685 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_687674 (CHEMBL1291058) |
|---|
| IC50 | >500000±n/a nM |
|---|
| Citation |  Zou, Y; Albohy, A; Sandbhor, M; Cairo, CW Inhibition of human neuraminidase 3 (NEU3) by C9-triazole derivatives of 2,3-didehydro-N-acetyl-neuraminic acid. Bioorg Med Chem Lett20:7529-33 (2010) [PubMed] Article Zou, Y; Albohy, A; Sandbhor, M; Cairo, CW Inhibition of human neuraminidase 3 (NEU3) by C9-triazole derivatives of 2,3-didehydro-N-acetyl-neuraminic acid. Bioorg Med Chem Lett20:7529-33 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Sialidase-3 |
|---|
| Name: | Sialidase-3 |
|---|
| Synonyms: | NEU3 | NEUR3_HUMAN | Sialidase 3 | Sialidase-3 |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 48257.99 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_461856 |
|---|
| Residue: | 428 |
|---|
| Sequence: | MEEVTTCSFNSPLFRQEDDRGITYRIPALLYIPPTHTFLAFAEKRSTRRDEDALHLVLRR
GLRIGQLVQWGPLKPLMEATLPGHRTMNPCPVWEQKSGCVFLFFICVRGHVTERQQIVSG
RNAARLCFIYSQDAGCSWSEVRDLTEEVIGSELKHWATFAVGPGHGIQLQSGRLVIPAYT
YYIPSWFFCFQLPCKTRPHSLMIYSDDLGVTWHHGRLIRPMVTVECEVAEVTGRAGHPVL
YCSARTPNRCRAEALSTDHGEGFQRLALSRQLCEPPHGCQGSVVSFRPLEIPHRCQDSSS
KDAPTIQQSSPGSSLRLEEEAGTPSESWLLYSHPTSRKQRVDLGIYLNQTPLEAACWSRP
WILHCGPCGYSDLAALEEEGLFGCLFECGTKQECEQIAFRLFTHREILSHLQGDCTSPGR
NPSQFKSN
|
|
|
|---|
| BDBM50331685 |
|---|
| n/a |
|---|
| Name | BDBM50331685 |
|---|
| Synonyms: | (2R,3R,4S)-3-(2-(4-ethoxy-1H-1,2,3-triazol-1-yl)acetamido)-4-hydroxy-2-((1R,2R)-1,2,3-trihydroxypropyl)-3,4-dihydro-2H-pyran-6-carboxylate | CHEMBL1290075 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C15H22N4O9 |
|---|
| Mol. Mass. | 402.3566 |
|---|
| SMILES | CCOc1cn(CC(=O)N[C@@H]2[C@@H](O)C=C(O[C@H]2[C@H](O)[C@H](O)CO)C(O)=O)nn1 |r,c:13| |
|---|
| Structure | 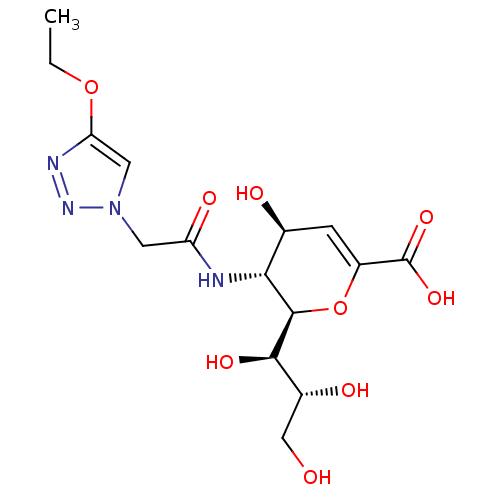
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Zou, Y; Albohy, A; Sandbhor, M; Cairo, CW Inhibition of human neuraminidase 3 (NEU3) by C9-triazole derivatives of 2,3-didehydro-N-acetyl-neuraminic acid. Bioorg Med Chem Lett20:7529-33 (2010) [PubMed] Article
Zou, Y; Albohy, A; Sandbhor, M; Cairo, CW Inhibition of human neuraminidase 3 (NEU3) by C9-triazole derivatives of 2,3-didehydro-N-acetyl-neuraminic acid. Bioorg Med Chem Lett20:7529-33 (2010) [PubMed] Article