| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Peroxisome proliferator-activated receptor alpha |
|---|
| Ligand | BDBM50176605 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_702774 (CHEMBL1655217) |
|---|
| EC50 | 12±n/a nM |
|---|
| Citation |  Ohashi, M; Oyama, T; Nakagome, I; Satoh, M; Nishio, Y; Nobusada, H; Hirono, S; Morikawa, K; Hashimoto, Y; Miyachi, H Design, synthesis, and structural analysis of phenylpropanoic acid-type PPAR¿-selective agonists: discovery of reversed stereochemistry-activity relationship. J Med Chem54:331-41 (2011) [PubMed] Article Ohashi, M; Oyama, T; Nakagome, I; Satoh, M; Nishio, Y; Nobusada, H; Hirono, S; Morikawa, K; Hashimoto, Y; Miyachi, H Design, synthesis, and structural analysis of phenylpropanoic acid-type PPAR¿-selective agonists: discovery of reversed stereochemistry-activity relationship. J Med Chem54:331-41 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Peroxisome proliferator-activated receptor alpha |
|---|
| Name: | Peroxisome proliferator-activated receptor alpha |
|---|
| Synonyms: | NR1C1 | Nuclear receptor subfamily 1 group C member 1 | PPAR | PPAR alpha/gamma | PPAR-alpha | PPARA | PPARA_HUMAN | Peroxisome Proliferator-Activated Receptor alpha | Peroxisome proliferator-activated receptor | Peroxisome proliferator-activated receptor alpha (PPAR alpha) |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 52222.08 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q07869 |
|---|
| Residue: | 468 |
|---|
| Sequence: | MVDTESPLCPLSPLEAGDLESPLSEEFLQEMGNIQEISQSIGEDSSGSFGFTEYQYLGSC
PGSDGSVITDTLSPASSPSSVTYPVVPGSVDESPSGALNIECRICGDKASGYHYGVHACE
GCKGFFRRTIRLKLVYDKCDRSCKIQKKNRNKCQYCRFHKCLSVGMSHNAIRFGRMPRSE
KAKLKAEILTCEHDIEDSETADLKSLAKRIYEAYLKNFNMNKVKARVILSGKASNNPPFV
IHDMETLCMAEKTLVAKLVANGIQNKEAEVRIFHCCQCTSVETVTELTEFAKAIPGFANL
DLNDQVTLLKYGVYEAIFAMLSSVMNKDGMLVAYGNGFITREFLKSLRKPFCDIMEPKFD
FAMKFNALELDDSDISLFVAAIICCGDRPGLLNVGHIEKMQEGIVHVLRLHLQSNHPDDI
FLFPKLLQKMADLRQLVTEHAQLVQIIKKTESDAALHPLLQEIYRDMY
|
|
|
|---|
| BDBM50176605 |
|---|
| n/a |
|---|
| Name | BDBM50176605 |
|---|
| Synonyms: | (S)-2-(3-((3-fluoro-4-(trifluoromethyl)benzamido)methyl)-4-methoxybenzyl)butanoic acid | CHEMBL202118 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H21F4NO4 |
|---|
| Mol. Mass. | 427.3894 |
|---|
| SMILES | CC[C@@H](Cc1ccc(OC)c(CNC(=O)c2ccc(c(F)c2)C(F)(F)F)c1)C(O)=O |
|---|
| Structure | 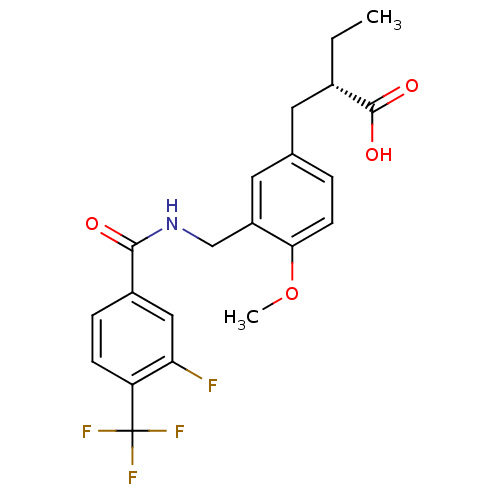
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ohashi, M; Oyama, T; Nakagome, I; Satoh, M; Nishio, Y; Nobusada, H; Hirono, S; Morikawa, K; Hashimoto, Y; Miyachi, H Design, synthesis, and structural analysis of phenylpropanoic acid-type PPAR¿-selective agonists: discovery of reversed stereochemistry-activity relationship. J Med Chem54:331-41 (2011) [PubMed] Article
Ohashi, M; Oyama, T; Nakagome, I; Satoh, M; Nishio, Y; Nobusada, H; Hirono, S; Morikawa, K; Hashimoto, Y; Miyachi, H Design, synthesis, and structural analysis of phenylpropanoic acid-type PPAR¿-selective agonists: discovery of reversed stereochemistry-activity relationship. J Med Chem54:331-41 (2011) [PubMed] Article