| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cathepsin S |
|---|
| Ligand | BDBM50335285 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_702783 (CHEMBL1655226) |
|---|
| Ki | 0.32±n/a nM |
|---|
| Citation |  Frizler, M; Lohr, F; Furtmann, N; Kläs, J; Gütschow, M Structural optimization of azadipeptide nitriles strongly increases association rates and allows the development of selective cathepsin inhibitors. J Med Chem54:396-400 (2011) [PubMed] Article Frizler, M; Lohr, F; Furtmann, N; Kläs, J; Gütschow, M Structural optimization of azadipeptide nitriles strongly increases association rates and allows the development of selective cathepsin inhibitors. J Med Chem54:396-400 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cathepsin S |
|---|
| Name: | Cathepsin S |
|---|
| Synonyms: | CATS_HUMAN | CTSS | Cathepsin S (Cat S) | cathepsin S preproprotein |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 37507.38 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P25774 |
|---|
| Residue: | 331 |
|---|
| Sequence: | MKRLVCVLLVCSSAVAQLHKDPTLDHHWHLWKKTYGKQYKEKNEEAVRRLIWEKNLKFVM
LHNLEHSMGMHSYDLGMNHLGDMTSEEVMSLMSSLRVPSQWQRNITYKSNPNRILPDSVD
WREKGCVTEVKYQGSCGACWAFSAVGALEAQLKLKTGKLVSLSAQNLVDCSTEKYGNKGC
NGGFMTTAFQYIIDNKGIDSDASYPYKAMDQKCQYDSKYRAATCSKYTELPYGREDVLKE
AVANKGPVSVGVDARHPSFFLYRSGVYYEPSCTQNVNHGVLVVGYGDLNGKEYWLVKNSW
GHNFGEEGYIRMARNKGNHCGIASFPSYPEI
|
|
|
|---|
| BDBM50335285 |
|---|
| n/a |
|---|
| Name | BDBM50335285 |
|---|
| Synonyms: | CHEMBL1651350 | N-{4-[5-(2-Thienyl)-1,2,4-oxadiazol-3-yl]phenylcarbamoyl}-leucyl-methylazalanine-nitrile | acs.jmedchem.1c00409_ST.407 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H25N7O3S |
|---|
| Mol. Mass. | 467.544 |
|---|
| SMILES | CC(C)C[C@H](NC(=O)Nc1ccc(cc1)-c1noc(n1)-c1cccs1)C(=O)N(C)N(C)C#N |r| |
|---|
| Structure | 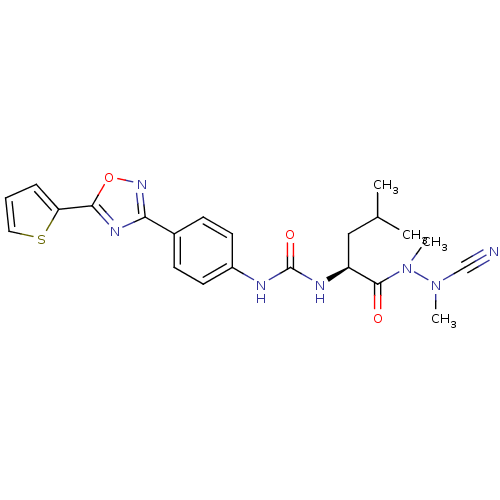
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Frizler, M; Lohr, F; Furtmann, N; Kläs, J; Gütschow, M Structural optimization of azadipeptide nitriles strongly increases association rates and allows the development of selective cathepsin inhibitors. J Med Chem54:396-400 (2011) [PubMed] Article
Frizler, M; Lohr, F; Furtmann, N; Kläs, J; Gütschow, M Structural optimization of azadipeptide nitriles strongly increases association rates and allows the development of selective cathepsin inhibitors. J Med Chem54:396-400 (2011) [PubMed] Article