| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Phospho-N-acetylmuramoyl-pentapeptide-transferase |
|---|
| Ligand | BDBM50386967 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_827497 (CHEMBL2051354) |
|---|
| IC50 | 8900±n/a nM |
|---|
| Citation |  Okamoto, K; Sakagami, M; Feng, F; Takahashi, F; Uotani, K; Togame, H; Takemoto, H; Ichikawa, S; Matsuda, A Synthesis of pacidamycin analogues via an Ugi-multicomponent reaction. Bioorg Med Chem Lett22:4810-5 (2012) [PubMed] Article Okamoto, K; Sakagami, M; Feng, F; Takahashi, F; Uotani, K; Togame, H; Takemoto, H; Ichikawa, S; Matsuda, A Synthesis of pacidamycin analogues via an Ugi-multicomponent reaction. Bioorg Med Chem Lett22:4810-5 (2012) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Phospho-N-acetylmuramoyl-pentapeptide-transferase |
|---|
| Name: | Phospho-N-acetylmuramoyl-pentapeptide-transferase |
|---|
| Synonyms: | MRAY_STAAU | mraY |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 35238.55 |
|---|
| Organism: | Staphylococcus aureus (strain MRSA252) |
|---|
| Description: | ChEMBL_827497 |
|---|
| Residue: | 321 |
|---|
| Sequence: | MIFVYALLALVITFVLVPVLIPTLKRMKFGQSIREEGPQSHMKKTGTPTMGGLTFLLSIV
ITSLVAIIFVDQANPIILLLFVTIGFGLIGFIDDYIIVVKKNNQGLTSKQKFLAQIGIAI
IFFVLSNVFHLVNFSTSIHIPFTNVAIPLSFAYVIFIVFWQVGFSNAVNLTDGLDGLATG
LSIIGFTMYAIMSFVLGETAIGIFCIIMLFALLGFLPYNINPAKVFMGDTGSLALGGIFA
TISIMLNQELSLIFIGLVFVIETLSVMLQVASFKLTGKRIFKMSPIHHHFELIGWSEWKV
VTVFWAVGLISGLIGLWIGVH
|
|
|
|---|
| BDBM50386967 |
|---|
| n/a |
|---|
| Name | BDBM50386967 |
|---|
| Synonyms: | CHEMBL2048832 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C40H48N10O13 |
|---|
| Mol. Mass. | 876.8683 |
|---|
| SMILES | C[C@H](NC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O)C(=O)N[C@H]([C@H](C)N(C)C(=O)[C@@H](Cc1cccc(O)c1)NC(=O)CN)C(=O)N\C=C1/O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O |r| |
|---|
| Structure | 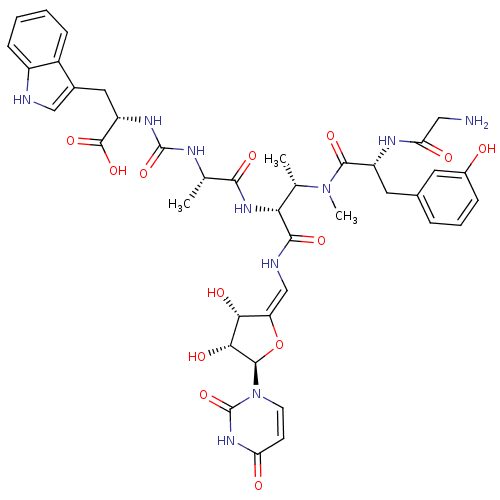
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Okamoto, K; Sakagami, M; Feng, F; Takahashi, F; Uotani, K; Togame, H; Takemoto, H; Ichikawa, S; Matsuda, A Synthesis of pacidamycin analogues via an Ugi-multicomponent reaction. Bioorg Med Chem Lett22:4810-5 (2012) [PubMed] Article
Okamoto, K; Sakagami, M; Feng, F; Takahashi, F; Uotani, K; Togame, H; Takemoto, H; Ichikawa, S; Matsuda, A Synthesis of pacidamycin analogues via an Ugi-multicomponent reaction. Bioorg Med Chem Lett22:4810-5 (2012) [PubMed] Article