| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Receptor-type tyrosine-protein phosphatase F |
|---|
| Ligand | BDBM50148911 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_848287 (CHEMBL2149376) |
|---|
| IC50 | 3800±n/a nM |
|---|
| Citation |  Ramírez-Espinosa, JJ; Rios, MY; López-Martínez, S; López-Vallejo, F; Medina-Franco, JL; Paoli, P; Camici, G; Navarrete-Vázquez, G; Ortiz-Andrade, R; Estrada-Soto, S Antidiabetic activity of some pentacyclic acid triterpenoids, role of PTP-1B: in vitro, in silico, and in vivo approaches. Eur J Med Chem46:2243-51 (2011) [PubMed] Article Ramírez-Espinosa, JJ; Rios, MY; López-Martínez, S; López-Vallejo, F; Medina-Franco, JL; Paoli, P; Camici, G; Navarrete-Vázquez, G; Ortiz-Andrade, R; Estrada-Soto, S Antidiabetic activity of some pentacyclic acid triterpenoids, role of PTP-1B: in vitro, in silico, and in vivo approaches. Eur J Med Chem46:2243-51 (2011) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Receptor-type tyrosine-protein phosphatase F |
|---|
| Name: | Receptor-type tyrosine-protein phosphatase F |
|---|
| Synonyms: | LAR | Leukocyte common antigen related | Leukocyte common antigen related (LAR) | PTPRF | PTPRF_HUMAN | Receptor-type tyrosine-protein phosphatase F | Receptor-type tyrosine-protein phosphatase F (LAR) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 212869.85 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P10586 |
|---|
| Residue: | 1907 |
|---|
| Sequence: | MAPEPAPGRTMVPLVPALVMLGLVAGAHGDSKPVFIKVPEDQTGLSGGVASFVCQATGEP
KPRITWMKKGKKVSSQRFEVIEFDDGAGSVLRIQPLRVQRDEAIYECTATNSLGEINTSA
KLSVLEEEQLPPGFPSIDMGPQLKVVEKARTATMLCAAGGNPDPEISWFKDFLPVDPATS
NGRIKQLRSGALQIESSEESDQGKYECVATNSAGTRYSAPANLYVRVRRVAPRFSIPPSS
QEVMPGGSVNLTCVAVGAPMPYVKWMMGAEELTKEDEMPVGRNVLELSNVVRSANYTCVA
ISSLGMIEATAQVTVKALPKPPIDLVVTETTATSVTLTWDSGNSEPVTYYGIQYRAAGTE
GPFQEVDGVATTRYSIGGLSPFSEYAFRVLAVNSIGRGPPSEAVRARTGEQAPSSPPRRV
QARMLSASTMLVQWEPPEEPNGLVRGYRVYYTPDSRRPPNAWHKHNTDAGLLTTVGSLLP
GITYSLRVLAFTAVGDGPPSPTIQVKTQQGVPAQPADFQAEVESDTRIQLSWLLPPQERI
IMYELVYWAAEDEDQQHKVTFDPTSSYTLEDLKPDTLYRFQLAARSDMGVGVFTPTIEAR
TAQSTPSAPPQKVMCVSMGSTTVRVSWVPPPADSRNGVITQYSVAYEAVDGEDRGRHVVD
GISREHSSWDLVGLEKWTEYRVWVRAHTDVGPGPESSPVLVRTDEDVPSGPPRKVEVEPL
NSTAVHVYWKLPVPSKQHGQIRGYQVTYVRLENGEPRGLPIIQDVMLAEAQWRPEESEDY
ETTISGLTPETTYSVTVAAYTTKGDGARSKPKIVTTTGAVPGRPTMMISTTAMNTALLQW
HPPKELPGELLGYRLQYCRADEARPNTIDFGKDDQHFTVTGLHKGTTYIFRLAAKNRAGL
GEEFEKEIRTPEDLPSGFPQNLHVTGLTTSTTELAWDPPVLAERNGRIISYTVVFRDINS
QQELQNITTDTRFTLTGLKPDTTYDIKVRAWTSKGSGPLSPSIQSRTMPVEQVFAKNFRV
AAAMKTSVLLSWEVPDSYKSAVPFKILYNGQSVEVDGHSMRKLIADLQPNTEYSFVLMNR
GSSAGGLQHLVSIRTAPDLLPHKPLPASAYIEDGRFDLSMPHVQDPSLVRWFYIVVVPID
RVGGSMLTPRWSTPEELELDELLEAIEQGGEEQRRRRRQAERLKPYVAAQLDVLPETFTL
GDKKNYRGFYNRPLSPDLSYQCFVLASLKEPMDQKRYASSPYSDEIVVQVTPAQQQEEPE
MLWVTGPVLAVILIILIVIAILLFKRKRTHSPSSKDEQSIGLKDSLLAHSSDPVEMRRLN
YQTPGMRDHPPIPITDLADNIERLKANDGLKFSQEYESIDPGQQFTWENSNLEVNKPKNR
YANVIAYDHSRVILTSIDGVPGSDYINANYIDGYRKQNAYIATQGPLPETMGDFWRMVWE
QRTATVVMMTRLEEKSRVKCDQYWPARGTETCGLIQVTLLDTVELATYTVRTFALHKSGS
SEKRELRQFQFMAWPDHGVPEYPTPILAFLRRVKACNPLDAGPMVVHCSAGVGRTGCFIV
IDAMLERMKHEKTVDIYGHVTCMRSQRNYMVQTEDQYVFIHEALLEAATCGHTEVPARNL
YAHIQKLGQVPPGESVTAMELEFKLLASSKAHTSRFISANLPCNKFKNRLVNIMPYELTR
VCLQPIRGVEGSDYINASFLDGYRQQKAYIATQGPLAESTEDFWRMLWEHNSTIIVMLTK
LREMGREKCHQYWPAERSARYQYFVVDPMAEYNMPQYILREFKVTDARDGQSRTIRQFQF
TDWPEQGVPKTGEGFIDFIGQVHKTKEQFGQDGPITVHCSAGVGRTGVFITLSIVLERMR
YEGVVDMFQTVKTLRTQRPAMVQTEDQYQLCYRAALEYLGSFDHYAT
|
|
|
|---|
| BDBM50148911 |
|---|
| n/a |
|---|
| Name | BDBM50148911 |
|---|
| Synonyms: | (3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hydroxyurs-12-en-28-oic acid | CHEMBL169 | Ursolic acid | malol | prunol | urson |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C30H48O3 |
|---|
| Mol. Mass. | 456.7003 |
|---|
| SMILES | C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9| |
|---|
| Structure | 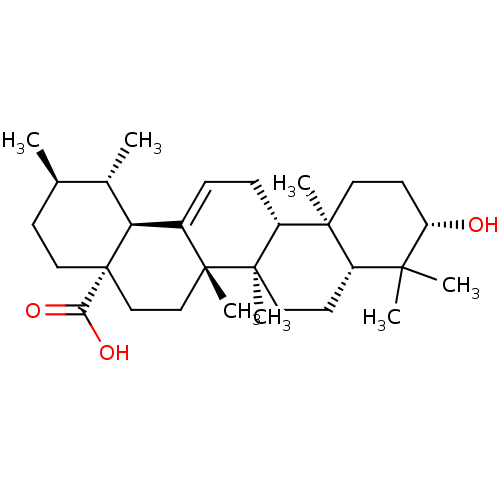
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Ramírez-Espinosa, JJ; Rios, MY; López-Martínez, S; López-Vallejo, F; Medina-Franco, JL; Paoli, P; Camici, G; Navarrete-Vázquez, G; Ortiz-Andrade, R; Estrada-Soto, S Antidiabetic activity of some pentacyclic acid triterpenoids, role of PTP-1B: in vitro, in silico, and in vivo approaches. Eur J Med Chem46:2243-51 (2011) [PubMed] Article
Ramírez-Espinosa, JJ; Rios, MY; López-Martínez, S; López-Vallejo, F; Medina-Franco, JL; Paoli, P; Camici, G; Navarrete-Vázquez, G; Ortiz-Andrade, R; Estrada-Soto, S Antidiabetic activity of some pentacyclic acid triterpenoids, role of PTP-1B: in vitro, in silico, and in vivo approaches. Eur J Med Chem46:2243-51 (2011) [PubMed] Article