| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Trifunctional purine biosynthetic protein adenosine-3 |
|---|
| Ligand | BDBM50393639 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_855845 (CHEMBL2161359) |
|---|
| IC50 | 2.03±n/a nM |
|---|
| Citation |  Wang, L; Cherian, C; Kugel Desmoulin, S; Mitchell-Ryan, S; Hou, Z; Matherly, LH; Gangjee, A Synthesis and biological activity of 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl regioisomers as inhibitors of de novo purine biosynthesis with selectivity for cellular uptake by high affinity folate receptors and the proton-coupled folate transporter over the reduced folate carrier. J Med Chem55:1758-70 (2012) [PubMed] Article Wang, L; Cherian, C; Kugel Desmoulin, S; Mitchell-Ryan, S; Hou, Z; Matherly, LH; Gangjee, A Synthesis and biological activity of 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl regioisomers as inhibitors of de novo purine biosynthesis with selectivity for cellular uptake by high affinity folate receptors and the proton-coupled folate transporter over the reduced folate carrier. J Med Chem55:1758-70 (2012) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Trifunctional purine biosynthetic protein adenosine-3 |
|---|
| Name: | Trifunctional purine biosynthetic protein adenosine-3 |
|---|
| Synonyms: | GAR Tfase | GAR transformylase | GART | Glycinamide ribonucleotide formyltransferase (GARFTase) | Glycinamide ribonucleotide transformylase (GAR Tfase) | PGFT | PRGS | PUR2_HUMAN | Thymidylate synthase/GAR transformylase/AICAR transformylase |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 107768.47 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P22102 |
|---|
| Residue: | 1010 |
|---|
| Sequence: | MAARVLIIGSGGREHTLAWKLAQSHHVKQVLVAPGNAGTACSEKISNTAISISDHTALAQ
FCKEKKIEFVVVGPEAPLAAGIVGNLRSAGVQCFGPTAEAAQLESSKRFAKEFMDRHGIP
TAQWKAFTKPEEACSFILSADFPALVVKASGLAAGKGVIVAKSKEEACKAVQEIMQEKAF
GAAGETIVIEELLDGEEVSCLCFTDGKTVAPMPPAQDHKRLLEGDGGPNTGGMGAYCPAP
QVSNDLLLKIKDTVLQRTVDGMQQEGTPYTGILYAGIMLTKNGPKVLEFNCRFGDPECQV
ILPLLKSDLYEVIQSTLDGLLCTSLPVWLENHTALTVVMASKGYPGDYTKGVEITGFPEA
QALGLEVFHAGTALKNGKVVTHGGRVLAVTAIRENLISALEEAKKGLAAIKFEGAIYRKD
VGFRAIAFLQQPRSLTYKESGVDIAAGNMLVKKIQPLAKATSRSGCKVDLGGFAGLFDLK
AAGFKDPLLASGTDGVGTKLKIAQLCNKHDTIGQDLVAMCVNDILAQGAEPLFFLDYFSC
GKLDLSVTEAVVAGIAKACGKAGCALLGGETAEMPDMYPPGEYDLAGFAVGAMERDQKLP
HLERITEGDVVVGIASSGLHSNGFSLVRKIVAKSSLQYSSPAPDGCGDQTLGDLLLTPTR
IYSHSLLPVLRSGHVKAFAHITGGGLLENIPRVLPEKLGVDLDAQTWRIPRVFSWLQQEG
HLSEEEMARTFNCGVGAVLVVSKEQTEQILRDIQQHKEEAWVIGSVVARAEGSPRVKVKN
LIESMQINGSVLKNGSLTNHFSFEKKKARVAVLISGTGSNLQALIDSTREPNSSAQIDIV
ISNKAAVAGLDKAERAGIPTRVINHKLYKNRVEFDSAIDLVLEEFSIDIVCLAGFMRILS
GPFVQKWNGKMLNIHPSLLPSFKGSNAHEQALETGVTVTGCTVHFVAEDVDAGQIILQEA
VPVKRGDTVATLSERVKLAEHKIFPAALQLVASGTVQLGENGKICWVKEE
|
|
|
|---|
| BDBM50393639 |
|---|
| n/a |
|---|
| Name | BDBM50393639 |
|---|
| Synonyms: | CHEMBL2158682 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C20H23N5O6S |
|---|
| Mol. Mass. | 461.492 |
|---|
| SMILES | Nc1nc2[nH]c(CCCCc3cc(cs3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| |
|---|
| Structure | 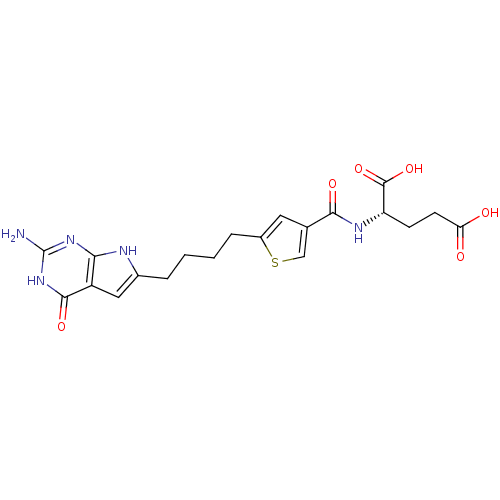
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Wang, L; Cherian, C; Kugel Desmoulin, S; Mitchell-Ryan, S; Hou, Z; Matherly, LH; Gangjee, A Synthesis and biological activity of 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl regioisomers as inhibitors of de novo purine biosynthesis with selectivity for cellular uptake by high affinity folate receptors and the proton-coupled folate transporter over the reduced folate carrier. J Med Chem55:1758-70 (2012) [PubMed] Article
Wang, L; Cherian, C; Kugel Desmoulin, S; Mitchell-Ryan, S; Hou, Z; Matherly, LH; Gangjee, A Synthesis and biological activity of 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl regioisomers as inhibitors of de novo purine biosynthesis with selectivity for cellular uptake by high affinity folate receptors and the proton-coupled folate transporter over the reduced folate carrier. J Med Chem55:1758-70 (2012) [PubMed] Article