| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cathepsin K |
|---|
| Ligand | BDBM50395241 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_860167 (CHEMBL2168708) |
|---|
| IC50 | 2±n/a nM |
|---|
| Citation |  Dossetter, AG; Beeley, H; Bowyer, J; Cook, CR; Crawford, JJ; Finlayson, JE; Heron, NM; Heyes, C; Highton, AJ; Hudson, JA; Jestel, A; Kenny, PW; Krapp, S; Martin, S; MacFaul, PA; McGuire, TM; Gutierrez, PM; Morley, AD; Morris, JJ; Page, KM; Ribeiro, LR; Sawney, H; Steinbacher, S; Smith, C; Vickers, M (1R,2R)-N-(1-cyanocyclopropyl)-2-(6-methoxy-1,3,4,5-tetrahydropyrido[4,3-b]indole-2-carbonyl)cyclohexanecarboxamide (AZD4996): a potent and highly selective cathepsin K inhibitor for the treatment of osteoarthritis. J Med Chem55:6363-74 (2012) [PubMed] Article Dossetter, AG; Beeley, H; Bowyer, J; Cook, CR; Crawford, JJ; Finlayson, JE; Heron, NM; Heyes, C; Highton, AJ; Hudson, JA; Jestel, A; Kenny, PW; Krapp, S; Martin, S; MacFaul, PA; McGuire, TM; Gutierrez, PM; Morley, AD; Morris, JJ; Page, KM; Ribeiro, LR; Sawney, H; Steinbacher, S; Smith, C; Vickers, M (1R,2R)-N-(1-cyanocyclopropyl)-2-(6-methoxy-1,3,4,5-tetrahydropyrido[4,3-b]indole-2-carbonyl)cyclohexanecarboxamide (AZD4996): a potent and highly selective cathepsin K inhibitor for the treatment of osteoarthritis. J Med Chem55:6363-74 (2012) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cathepsin K |
|---|
| Name: | Cathepsin K |
|---|
| Synonyms: | CATK_HUMAN | CTSK | CTSO | CTSO2 | Cathepsin O | Cathepsin O2 | Cathepsin X |
|---|
| Type: | Enzyme |
|---|
| Mol. Mass.: | 36975.68 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P43235 |
|---|
| Residue: | 329 |
|---|
| Sequence: | MWGLKVLLLPVVSFALYPEEILDTHWELWKKTHRKQYNNKVDEISRRLIWEKNLKYISIH
NLEASLGVHTYELAMNHLGDMTSEEVVQKMTGLKVPLSHSRSNDTLYIPEWEGRAPDSVD
YRKKGYVTPVKNQGQCGSCWAFSSVGALEGQLKKKTGKLLNLSPQNLVDCVSENDGCGGG
YMTNAFQYVQKNRGIDSEDAYPYVGQEESCMYNPTGKAAKCRGYREIPEGNEKALKRAVA
RVGPVSVAIDASLTSFQFYSKGVYYDESCNSDNLNHAVLAVGYGIQKGNKHWIIKNSWGE
NWGNKGYILMARNKNNACGIANLASFPKM
|
|
|
|---|
| BDBM50395241 |
|---|
| n/a |
|---|
| Name | BDBM50395241 |
|---|
| Synonyms: | CHEMBL2163585 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C24H25N5O2 |
|---|
| Mol. Mass. | 415.4876 |
|---|
| SMILES | O=C(NC1(CC1)C#N)[C@@H]1CCCC[C@H]1C(=O)N1CCc2[nH]c3c(cccc3c2C1)C#N |r| |
|---|
| Structure | 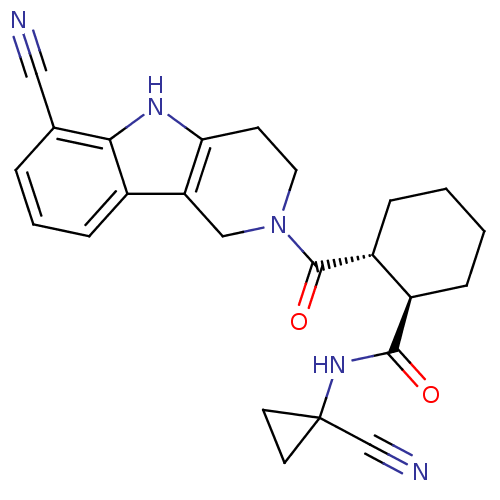
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Dossetter, AG; Beeley, H; Bowyer, J; Cook, CR; Crawford, JJ; Finlayson, JE; Heron, NM; Heyes, C; Highton, AJ; Hudson, JA; Jestel, A; Kenny, PW; Krapp, S; Martin, S; MacFaul, PA; McGuire, TM; Gutierrez, PM; Morley, AD; Morris, JJ; Page, KM; Ribeiro, LR; Sawney, H; Steinbacher, S; Smith, C; Vickers, M (1R,2R)-N-(1-cyanocyclopropyl)-2-(6-methoxy-1,3,4,5-tetrahydropyrido[4,3-b]indole-2-carbonyl)cyclohexanecarboxamide (AZD4996): a potent and highly selective cathepsin K inhibitor for the treatment of osteoarthritis. J Med Chem55:6363-74 (2012) [PubMed] Article
Dossetter, AG; Beeley, H; Bowyer, J; Cook, CR; Crawford, JJ; Finlayson, JE; Heron, NM; Heyes, C; Highton, AJ; Hudson, JA; Jestel, A; Kenny, PW; Krapp, S; Martin, S; MacFaul, PA; McGuire, TM; Gutierrez, PM; Morley, AD; Morris, JJ; Page, KM; Ribeiro, LR; Sawney, H; Steinbacher, S; Smith, C; Vickers, M (1R,2R)-N-(1-cyanocyclopropyl)-2-(6-methoxy-1,3,4,5-tetrahydropyrido[4,3-b]indole-2-carbonyl)cyclohexanecarboxamide (AZD4996): a potent and highly selective cathepsin K inhibitor for the treatment of osteoarthritis. J Med Chem55:6363-74 (2012) [PubMed] Article