

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | ALK tyrosine kinase receptor | ||
| Ligand | BDBM50396267 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_863887 (CHEMBL2176371) | ||
| IC50 | 3±n/a nM | ||
| Citation |  Lewis, RT; Bode, CM; Choquette, DM; Potashman, M; Romero, K; Stellwagen, JC; Teffera, Y; Moore, E; Whittington, DA; Chen, H; Epstein, LF; Emkey, R; Andrews, PS; Yu, VL; Saffran, DC; Xu, M; Drew, A; Merkel, P; Szilvassy, S; Brake, RL The discovery and optimization of a novel class of potent, selective, and orally bioavailable anaplastic lymphoma kinase (ALK) inhibitors with potential utility for the treatment of cancer. J Med Chem55:6523-40 (2012) [PubMed] Article Lewis, RT; Bode, CM; Choquette, DM; Potashman, M; Romero, K; Stellwagen, JC; Teffera, Y; Moore, E; Whittington, DA; Chen, H; Epstein, LF; Emkey, R; Andrews, PS; Yu, VL; Saffran, DC; Xu, M; Drew, A; Merkel, P; Szilvassy, S; Brake, RL The discovery and optimization of a novel class of potent, selective, and orally bioavailable anaplastic lymphoma kinase (ALK) inhibitors with potential utility for the treatment of cancer. J Med Chem55:6523-40 (2012) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| ALK tyrosine kinase receptor | |||
| Name: | ALK tyrosine kinase receptor | ||
| Synonyms: | ALK | ALK tyrosine kinase receptor (ALK) | ALK_HUMAN | Anaplastic lymphoma kinase | CD_antigen: CD246 | ||
| Type: | Protein | ||
| Mol. Mass.: | 176453.10 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | Q9UM73 | ||
| Residue: | 1620 | ||
| Sequence: |
| ||
| BDBM50396267 | |||
| n/a | |||
| Name | BDBM50396267 | ||
| Synonyms: | CHEMBL2172301 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C33H43ClFN5O3 | ||
| Mol. Mass. | 612.178 | ||
| SMILES | CC(C)NC(=O)[C@H]1CC[C@H](CC1)n1c(NC(=O)c2cc(F)cc(Cl)c2)nc2ccc(CN3CCC(CC3)C(C)(C)O)cc12 |r,wU:9.12,6.5,(20.95,-11.71,;19.45,-11.39,;18.97,-9.93,;18.42,-12.54,;16.91,-12.22,;15.88,-13.37,;16.43,-10.76,;14.93,-10.44,;14.45,-8.98,;15.48,-7.84,;16.98,-8.14,;17.46,-9.61,;14.99,-6.38,;15.9,-5.12,;17.44,-5.11,;18.2,-3.77,;17.42,-2.44,;19.74,-3.76,;20.49,-2.42,;22.03,-2.41,;22.79,-1.07,;22.81,-3.74,;22.05,-5.08,;22.83,-6.41,;20.51,-5.09,;14.98,-3.87,;13.51,-4.36,;12.17,-3.59,;10.84,-4.36,;10.84,-5.91,;9.51,-6.67,;8.18,-5.9,;8.18,-4.36,;6.86,-3.59,;5.52,-4.36,;5.52,-5.9,;6.85,-6.68,;4.18,-3.58,;3.41,-2.24,;4.95,-2.23,;2.85,-4.34,;12.18,-6.68,;13.52,-5.91,)| | ||
| Structure | 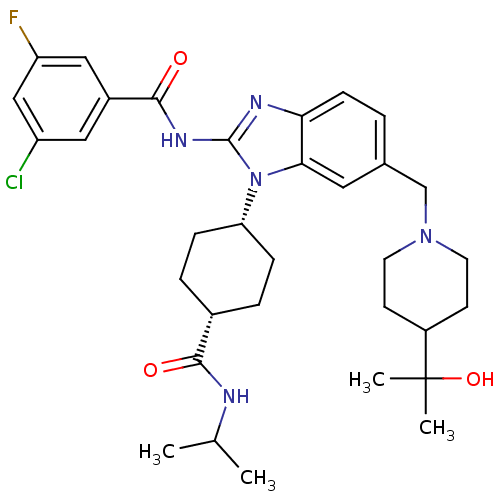
| ||