| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Histone-lysine N-methyltransferase, H3 lysine-79 specific |
|---|
| Ligand | BDBM50009672 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_863118 (CHEMBL2175616) |
|---|
| Ki | 160±n/a nM |
|---|
| Citation |  Anglin, JL; Deng, L; Yao, Y; Cai, G; Liu, Z; Jiang, H; Cheng, G; Chen, P; Dong, S; Song, Y Synthesis and structure-activity relationship investigation of adenosine-containing inhibitors of histone methyltransferase DOT1L. J Med Chem55:8066-74 (2012) [PubMed] Article Anglin, JL; Deng, L; Yao, Y; Cai, G; Liu, Z; Jiang, H; Cheng, G; Chen, P; Dong, S; Song, Y Synthesis and structure-activity relationship investigation of adenosine-containing inhibitors of histone methyltransferase DOT1L. J Med Chem55:8066-74 (2012) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Histone-lysine N-methyltransferase, H3 lysine-79 specific |
|---|
| Name: | Histone-lysine N-methyltransferase, H3 lysine-79 specific |
|---|
| Synonyms: | 2.1.1.43 | DOT1-like protein | DOT1-like protein (Dot1L) | DOT1L | DOT1L_HUMAN | H3-K79-HMTase | Histone H3-K79 methyltransferase | Histone H3-K79 methyltransferase (DOT1L) | Histone Methyltransferase DOT1L | Histone-lysine N-methyltransferase, H3 lysine-79 specific (DOT1L) | KIAA1814 | KMT4 | Lysine N-methyltransferase 4 |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 184911.91 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q8TEK3 |
|---|
| Residue: | 1537 |
|---|
| Sequence: | MGEKLELRLKSPVGAEPAVYPWPLPVYDKHHDAAHEIIETIRWVCEEIPDLKLAMENYVL
IDYDTKSFESMQRLCDKYNRAIDSIHQLWKGTTQPMKLNTRPSTGLLRHILQQVYNHSVT
DPEKLNNYEPFSPEVYGETSFDLVAQMIDEIKMTDDDLFVDLGSGVGQVVLQVAAATNCK
HHYGVEKADIPAKYAETMDREFRKWMKWYGKKHAEYTLERGDFLSEEWRERIANTSVIFV
NNFAFGPEVDHQLKERFANMKEGGRIVSSKPFAPLNFRINSRNLSDIGTIMRVVELSPLK
GSVSWTGKPVSYYLHTIDRTILENYFSSLKNPKLREEQEAARRRQQRESKSNAATPTKGP
EGKVAGPADAPMDSGAEEEKAGAATVKKPSPSKARKKKLNKKGRKMAGRKRGRPKKMNTA
NPERKPKKNQTALDALHAQTVSQTAASSPQDAYRSPHSPFYQLPPSVQRHSPNPLLVAPT
PPALQKLLESFKIQYLQFLAYTKTPQYKASLQELLGQEKEKNAQLLGAAQQLLSHCQAQK
EEIRRLFQQKLDELGVKALTYNDLIQAQKEISAHNQQLREQSEQLEQDNRALRGQSLQLL
KARCEELQLDWATLSLEKLLKEKQALKSQISEKQRHCLELQISIVELEKSQRQQELLQLK
SCVPPDDALSLHLRGKGALGRELEPDASRLHLELDCTKFSLPHLSSMSPELSMNGQAAGY
ELCGVLSRPSSKQNTPQYLASPLDQEVVPCTPSHVGRPRLEKLSGLAAPDYTRLSPAKIV
LRRHLSQDHTVPGRPAASELHSRAEHTKENGLPYQSPSVPGSMKLSPQDPRPLSPGALQL
AGEKSSEKGLRERAYGSSGELITSLPISIPLSTVQPNKLPVSIPLASVVLPSRAERARST
PSPVLQPRDPSSTLEKQIGANAHGAGSRSLALAPAGFSYAGSVAISGALAGSPASLTPGA
EPATLDESSSSGSLFATVGSRSSTPQHPLLLAQPRNSLPASPAHQLSSSPRLGGAAQGPL
PEASKGDLPSDSGFSDPESEAKRRIVFTITTGAGSAKQSPSSKHSPLTASARGDCVPSHG
QDSRRRGRRKRASAGTPSLSAGVSPKRRALPSVAGLFTQPSGSPLNLNSMVSNINQPLEI
TAISSPETSLKSSPVPYQDHDQPPVLKKERPLSQTNGAHYSPLTSDEEPGSEDEPSSARI
ERKIATISLESKSPPKTLENGGGLAGRKPAPAGEPVNSSKWKSTFSPISDIGLAKSADSP
LQASSALSQNSLFTFRPALEEPSADAKLAAHPRKGFPGSLSGADGLSPGTNPANGCTFGG
GLAADLSLHSFSDGASLPHKGPEAAGLSSPLSFPSQRGKEGSDANPFLSKRQLDGLAGLK
GEGSRGKEAGEGGLPLCGPTDKTPLLSGKAAKARDREVDLKNGHNLFISAAAVPPGSLLS
GPGLAPAASSAGGAASSAQTHRSFLGPFPPGPQFALGPMSLQANLGSVAGSSVLQSLFSS
VPAAAGLVHVSSAATRLTNSHAMGSFSGVAGGTVGGN
|
|
|
|---|
| BDBM50009672 |
|---|
| n/a |
|---|
| Name | BDBM50009672 |
|---|
| Synonyms: | AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocysteine | S-(5'-deoxyadenosin-5'-yl)-L-homocysteine | S-[1-(adenin-9-yl)-1,5-dideoxy-beta-D-ribofuranos-5-yl]-L-homocysteine | S-adenosyl-L-homocysteine | SAH | US8895245, S-Adenosyl-L-homocysteine (SAH) | US9175331, 1 | US9333217, S-Adenosyl-L-homocysteine (SAH) |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C14H20N6O5S |
|---|
| Mol. Mass. | 384.411 |
|---|
| SMILES | N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |
|---|
| Structure | 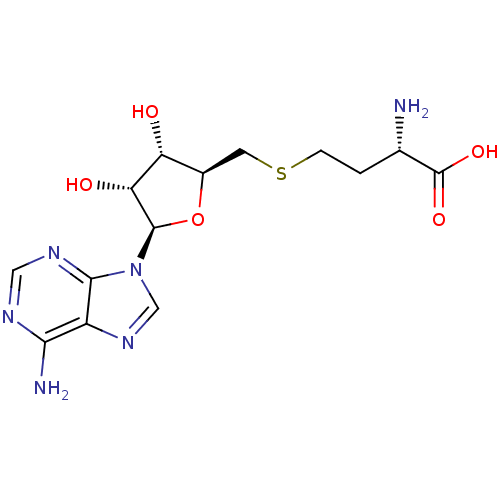
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Anglin, JL; Deng, L; Yao, Y; Cai, G; Liu, Z; Jiang, H; Cheng, G; Chen, P; Dong, S; Song, Y Synthesis and structure-activity relationship investigation of adenosine-containing inhibitors of histone methyltransferase DOT1L. J Med Chem55:8066-74 (2012) [PubMed] Article
Anglin, JL; Deng, L; Yao, Y; Cai, G; Liu, Z; Jiang, H; Cheng, G; Chen, P; Dong, S; Song, Y Synthesis and structure-activity relationship investigation of adenosine-containing inhibitors of histone methyltransferase DOT1L. J Med Chem55:8066-74 (2012) [PubMed] Article