| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2A6 |
|---|
| Ligand | BDBM50401421 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_883344 (CHEMBL2214080) |
|---|
| IC50 | >10000±n/a nM |
|---|
| Citation |  Bavetsias, V; Crumpler, S; Sun, C; Avery, S; Atrash, B; Faisal, A; Moore, AS; Kosmopoulou, M; Brown, N; Sheldrake, PW; Bush, K; Henley, A; Box, G; Valenti, M; de Haven Brandon, A; Raynaud, FI; Workman, P; Eccles, SA; Bayliss, R; Linardopoulos, S; Blagg, J Optimization of imidazo[4,5-b]pyridine-based kinase inhibitors: identification of a dual FLT3/Aurora kinase inhibitor as an orally bioavailable preclinical development candidate for the treatment of acute myeloid leukemia. J Med Chem55:8721-34 (2012) [PubMed] Article Bavetsias, V; Crumpler, S; Sun, C; Avery, S; Atrash, B; Faisal, A; Moore, AS; Kosmopoulou, M; Brown, N; Sheldrake, PW; Bush, K; Henley, A; Box, G; Valenti, M; de Haven Brandon, A; Raynaud, FI; Workman, P; Eccles, SA; Bayliss, R; Linardopoulos, S; Blagg, J Optimization of imidazo[4,5-b]pyridine-based kinase inhibitors: identification of a dual FLT3/Aurora kinase inhibitor as an orally bioavailable preclinical development candidate for the treatment of acute myeloid leukemia. J Med Chem55:8721-34 (2012) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2A6 |
|---|
| Name: | Cytochrome P450 2A6 |
|---|
| Synonyms: | 1,4-cineole 2-exo-monooxygenase | 1.14.13.- | CP2A6_HUMAN | CYP2A3 | CYP2A6 | CYPIIA6 | Coumarin 7-hydroxylase | Cytochrome P450 2A6 | Cytochrome P450 IIA3 | Cytochrome P450(I) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 56514.34 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P11509 |
|---|
| Residue: | 494 |
|---|
| Sequence: | MLASGMLLVALLVCLTVMVLMSVWQQRKSKGKLPPGPTPLPFIGNYLQLNTEQMYNSLMK
ISERYGPVFTIHLGPRRVVVLCGHDAVREALVDQAEEFSGRGEQATFDWVFKGYGVVFSN
GERAKQLRRFSIATLRDFGVGKRGIEERIQEEAGFLIDALRGTGGANIDPTFFLSRTVSN
VISSIVFGDRFDYKDKEFLSLLRMMLGIFQFTSTSTGQLYEMFSSVMKHLPGPQQQAFQL
LQGLEDFIAKKVEHNQRTLDPNSPRDFIDSFLIRMQEEEKNPNTEFYLKNLVMTTLNLFI
GGTETVSTTLRYGFLLLMKHPEVEAKVHEEIDRVIGKNRQPKFEDRAKMPYMEAVIHEIQ
RFGDVIPMSLARRVKKDTKFRDFFLPKGTEVYPMLGSVLRDPSFFSNPQDFNPQHFLNEK
GQFKKSDAFVPFSIGKRNCFGEGLARMELFLFFTTVMQNFRLKSSQSPKDIDVSPKHVGF
ATIPRNYTMSFLPR
|
|
|
|---|
| BDBM50401421 |
|---|
| n/a |
|---|
| Name | BDBM50401421 |
|---|
| Synonyms: | CHEMBL2207503 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C22H23Cl2N7 |
|---|
| Mol. Mass. | 456.371 |
|---|
| SMILES | Cc1nn(C)cc1-c1nc2ncc(Cl)c(N3CCN(Cc4ccc(Cl)cc4)CC3)c2[nH]1 |
|---|
| Structure | 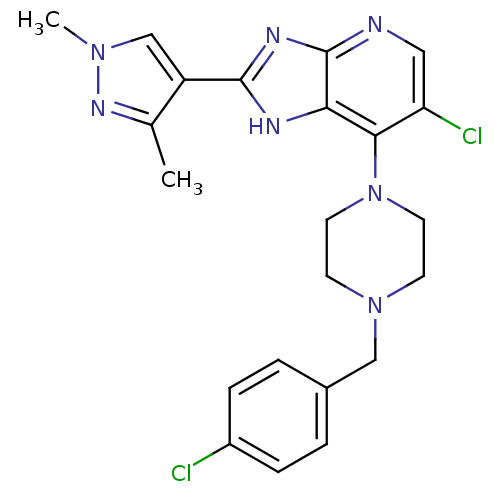
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Bavetsias, V; Crumpler, S; Sun, C; Avery, S; Atrash, B; Faisal, A; Moore, AS; Kosmopoulou, M; Brown, N; Sheldrake, PW; Bush, K; Henley, A; Box, G; Valenti, M; de Haven Brandon, A; Raynaud, FI; Workman, P; Eccles, SA; Bayliss, R; Linardopoulos, S; Blagg, J Optimization of imidazo[4,5-b]pyridine-based kinase inhibitors: identification of a dual FLT3/Aurora kinase inhibitor as an orally bioavailable preclinical development candidate for the treatment of acute myeloid leukemia. J Med Chem55:8721-34 (2012) [PubMed] Article
Bavetsias, V; Crumpler, S; Sun, C; Avery, S; Atrash, B; Faisal, A; Moore, AS; Kosmopoulou, M; Brown, N; Sheldrake, PW; Bush, K; Henley, A; Box, G; Valenti, M; de Haven Brandon, A; Raynaud, FI; Workman, P; Eccles, SA; Bayliss, R; Linardopoulos, S; Blagg, J Optimization of imidazo[4,5-b]pyridine-based kinase inhibitors: identification of a dual FLT3/Aurora kinase inhibitor as an orally bioavailable preclinical development candidate for the treatment of acute myeloid leukemia. J Med Chem55:8721-34 (2012) [PubMed] Article