

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Reaction Details | |||
|---|---|---|---|
 | Report a problem with these data | ||
| Target | Cathepsin S | ||
| Ligand | BDBM50401764 | ||
| Substrate/Competitor | n/a | ||
| Meas. Tech. | ChEMBL_885153 (CHEMBL2213244) | ||
| IC50 | 3±n/a nM | ||
| Citation |  Moss, N; Xiong, Z; Burke, M; Cogan, D; Gao, DA; Haverty, K; Heim-Riether, A; Hickey, ER; Nagaraja, R; Netherton, M; O'Shea, K; Ramsden, P; Schwartz, R; Shih, DT; Ward, Y; Young, E; Zhang, Q Exploration of cathepsin S inhibitors characterized by a triazole P1-P2 amide replacement. Bioorg Med Chem Lett22:7189-93 (2012) [PubMed] Article Moss, N; Xiong, Z; Burke, M; Cogan, D; Gao, DA; Haverty, K; Heim-Riether, A; Hickey, ER; Nagaraja, R; Netherton, M; O'Shea, K; Ramsden, P; Schwartz, R; Shih, DT; Ward, Y; Young, E; Zhang, Q Exploration of cathepsin S inhibitors characterized by a triazole P1-P2 amide replacement. Bioorg Med Chem Lett22:7189-93 (2012) [PubMed] Article | ||
| More Info.: | Get all data from this article, Assay Method | ||
| Cathepsin S | |||
| Name: | Cathepsin S | ||
| Synonyms: | CATS_HUMAN | CTSS | Cathepsin S (Cat S) | cathepsin S preproprotein | ||
| Type: | Protein | ||
| Mol. Mass.: | 37507.38 | ||
| Organism: | Homo sapiens (Human) | ||
| Description: | P25774 | ||
| Residue: | 331 | ||
| Sequence: |
| ||
| BDBM50401764 | |||
| n/a | |||
| Name | BDBM50401764 | ||
| Synonyms: | CHEMBL2207564 | ||
| Type | Small organic molecule | ||
| Emp. Form. | C19H25N5OS | ||
| Mol. Mass. | 371.5 | ||
| SMILES | C[C@H](C#N)n1cc(nn1)[C@@](C)(NC(=O)c1ccsc1)[C@H]1CC[C@H](C)CC1 |r,wU:9.10,22.24,wD:9.11,19.21,1.0,(18.92,-16.58,;18.21,-17.95,;19.04,-19.25,;19.87,-20.55,;16.66,-18.02,;15.81,-19.31,;14.34,-18.89,;14.26,-17.36,;15.7,-16.81,;13.13,-19.85,;12.34,-21.18,;11.79,-19.08,;10.45,-19.85,;10.45,-21.4,;9.23,-18.92,;7.75,-19.36,;6.87,-18.09,;7.81,-16.86,;9.27,-17.38,;13.88,-21.18,;13.11,-22.5,;13.86,-23.82,;15.39,-23.83,;16.15,-25.17,;16.16,-22.51,;15.41,-21.18,)| | ||
| Structure | 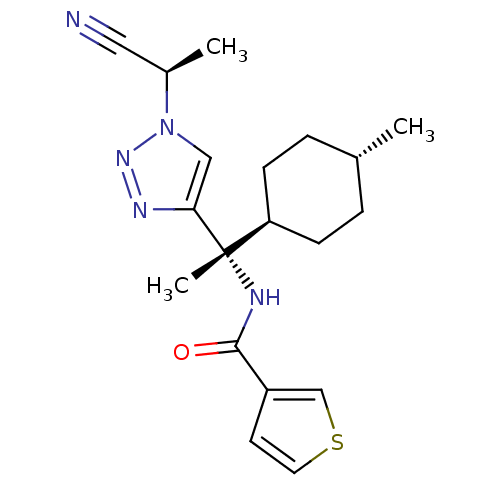
| ||